January 2026: New or Expanded Approvals for: GLP-1 Weight Loss, Birth Control Implant, Lung Disease, Menkes, Gaucher; Omeprazole Research and Case Studies; Rx-OTC Switches; Medication Tips
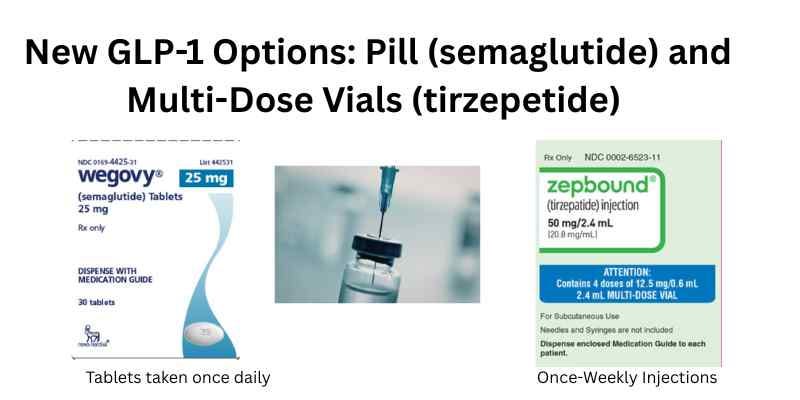
GLP-1 New Products: Wegovy (semaglutide) Tablets and Multi-Dose Vials of Mounjaro and Zepbound (tirzepatide)
Reuters reports that Novo Nordisk’s new Wegovy (semaglutide) pill is a hit, with total prescriptions filled during its first full week on the market topping 18,000. This compares with only about 1,000 prescriptions filled for the traditional pen injection formulation of Wegovy. Eli Lilly recently received approval for the multi-dose vial formulations of Zepbound (tirzepatide) and Mounjaro (tirzepatide). This is the first time the brand name (not compounded) GLP-1 formulations are available in multi-dose vials. Check the Reuters link below for a graph showing share of prescriptions for Wegovy pill, Wegovy pen, Zepbound pen, and Zepbound vials. Wegovy once-daily pill formulation was approved in December 2025; it costs less than the pen, with a price as low as $25 with insurance or $149 (depending on dose strength) without insurance.
Reuters: Tracking Wegovy Pill Prescriptions First Week of Launch
ABC News: Wegovy Daily Pill Costs
Dr. Lucy McBride: Are GLP-1 Pills as Good as the Shots? What’s Missing from the Debate
Lilly’s GLP-1 Orforglipron Weight Loss Pill
Eli Lilly is working on its own GLP-1 weight loss pill and expects its orforglipron pill to receive FDA approval for obesity by April. The medication is fast tracked for approval, having received an FDA priority review voucher in November 2025. The pills will be less expensive than Lilly’s Mounjaro or Zepbound pens; according to Lilly’s press release, LillyDirect* will charge $149 for the lowest dose and higher doses will cost up to $399. While Novo’s Wegovy pill needs to be taken in the morning on an empty stomach, 30 minutes before eating, drinking or taking other medications, Lilly says its pill has no such restrictions. Orforglipron’s application as a type 2 diabetes treatment will be filed with the FDA later this year.
Lilly press release: What to know about orforglipron
Kelo (Thomson Reuters) Lilly Confident in Weight Loss Pill Supply
*It is a controversial and emerging trend for pharma companies to both make and prescribe their own medications.
Expanded Approval for Long-Lasting Birth Control Implant
Organon’s Nexplanon (etonogestrel), a rod-shaped contraceptive device that is implanted just under the skin in the upper arm, received FDA approval that allows for extending its use for up to five-years instead of just three. The label was updated with clinical trials data that supporting its effectiveness and safety for the longer period of use. The label also has a new Risk Evaluation and Mitigation Strategy (REMS) program that aims to mitigate the risk of complications (such as infection, bleeding, pain) due to improper insertion and removal of the device. The REMS requires that healthcare providers receive training and certification. The Nexplanon FAERS Report Summary at AskaPatient lists more than 5,000 device-related complication issues, including more than 2,000 reports of device dislocation (most reports submitted by Organon). Site-specific procedural complications of Nexplanon were identified in the FDA’s “Potential Signs of Serious Risk Identified by the FDA Adverse Event Reporting System (FAERS)” for October-December 2024.
Etonogestrel is a progestin-only ingredient that prevents pregnancy by preventing ovulation. Nexplanon was launched in 2010 with improved safety features (such as preloaded applicator and ability to be seen on X-rays) and was originally approved as the brand Implanon in 2001.
Contemporary OB-GYN: FDA approves-5-year-use-for-etonogestrel-implant-68-mg-contraceptive
Safety Labeling Update for Nexplanon
Patient Experiences with Nexplanon
Summary of MedWatch Voluntary Reports for Nexplanon (240 reports of device complications for 1,577 reports submitted voluntarily, mainly by consumers)
Rare Disease: FDA Approves First Treatment for Children With Menkes Disease
The FDA approved the first-ever treatment for Menkes disease: Zydus Life Sciences’ Zycubo (copper histidinate) daily subcutaneous injection for pediatric patients. Menkes disease is a neurodegenerative disorder affecting boys, caused by a X-linked genetic defect that impairs a child’s ability to absorb dietary copper. The medication serves as a way to replace the copper without having it go through the gastrointestinal tract. In clinical trials, the results were impressive: a median survival rate of 177.1 months for the early treatment Zycubo cohort compared with only 17.6 months for the untreated external cohort.
Symptoms of Menkes include sparse and de-pigmented hair, seizures and developmental delays. If left untreated, many patients die between the ages of two and three years. The disease prevalence ranges from 1 in 34,810 to as high as 1 in 8,664 live male births.
FDA Press Release on First Treatment for Menkes Disease
Rare Disease: FDA Expands Treatment Approval for Gaucher Disease
The FDA expanded the approval of Sanofi’s Cerezyme (imiglucerase) intravenous infusion enzyme replacement therapy to treat non-neurological symptoms in patients with Type 3 Gaucher disease, which is the rarest and most serious type. Previously it had only been approved for Type 1, which is the most common form and does not cause neurological symptoms. Gaucher, a rare disease affecting about one in every 50,000 people in the world population and up to one in 500 people in people of Ashkenazi (eastern and central European) Jewish heritage. It is caused by genetic mutations that lead to a deficiency of the glucocerebrosidase (GCase) enzyme.
Symptoms including enlarged organs, blood abnormalities, and bone disease. Those with type 3 also experience neurological complications. Cerezyme is also now approved for all ages, from birth and up. Cerezyme was originally approved in 1994. A label was updated with new safety warnings about hypersensitivity reactions and recommends infusion dosage modifications based on these infusion-related reactions.
FDA Cerezyme Updated Label and Safety Information
Read more about Gaucher Disease at Medline Plus.
Gaucher Disease News: Cerezyme Becomes First FDA-Approved Therapy for Type 3 Gaucher Disease
New Treatment for Progressive Pulmonary Fibrosis Lung Disease
The FDA approved Boehringer Ingelheim’s Jascayd (nerandomilast) tablets to treat adults with progressive pulmonary fibrosis (PPF). Jascayd was previously approved to treat idiopathic pulmonary fibrosis (IFP) in adults. PPF is a chronic disease characterized by gradual, irreversible scarring of the lungs, which can lead to progressive breathing difficulties. PPF is an umbrella term that can describe progressive lung scarring in many interstitial lung diseases.
FDA Press Release on Jascayd
Converting More Prescription Drugs to Nonprescription Drugs
The two most recent prescription drugs to become available over-the-counter (OTC) in the U.S. were the O-pill (norgestrel) oral contraceptive and Narcan (naloxone) opioid overdose nasal spray, which both were approved for OTC in 2023. FDA Chair Marty Makary would like to see more prescription medicines available over-the-counter (or without a prescription).
A new rule that went into effect in May 2025 could help make this happen by creating an “additional condition for nonprescription use” protocol, which means that a person may need to consult with a pharmacist or answer questions on an automated telephone system or an app before being allowed to purchase a medication. In the United States, drugs are classified as either “Over-the-counter” or “Prescription only,” but that is not the case in other parts of the world, where there may be three or more approval types for access to medications.
Forbes: Trump Administration Says it Wants More OTC Meds But So Far No Action
What do you think? Should new categories of prescription drugs be made available to purchase in the U.S. without a prescription? If so, do you have a suggestion for a medication that would be a good candidate to make the switch? If so, add it to the comment box before clicking “Vote.” We’ll share the suggested answers in a future newsletter. Link to the poll here.
PPI Medications (like Omeprazole) and Research on Adverse Effects
Researchers at Sweden’s Karolinska Institute and other institutions in Denmark, Finland, Iceland, and Norway found no association between long-term proton pump inhibitor (PPI) use and stomach cancer. Previously, other studies suggested there was an association. PPIs are drugs taken for GERD (gastro-esophageal reflux disease) like omeprazole (Prilosec brand name), esomeprazole (Nexium), and lansoprazole (Prevacid) that block the secretion of stomach acid. In the large population-based study, which utilized Nordic country health care registries, researchers adjusted the data to address earlier studies’ known weaknesses, such as the fact that many people beginning to get stomach cancer symptoms would begin to take PPIs, but that did not mean that the patients’ cancer was caused by the PPIs.
As the authors point out, long-term proton pump inhibitor use is connected to serious side effects and increases the risk of some other potentially serious conditions such as Clostridium difficile associated diarrhea, osteoporosis, and vitamin or electrolyte malabsorption. “This highlights the need to balance the benefits and disadvantages of such use and to regularly reassess the need for continued proton pump inhibitor treatment.”
The research was published in The British Medical Journal (BMJ). British Medical Journal: Long term use of proton pump inhibitors and risk of stomach cancer: population based case-control study in five Nordic countries
Serious Adverse Effects of PPIs
While the connection of PPIs to dementia or cognitive decline has also been discounted, omeprazole has been linked to kidney injury. In October 2023, we reported that AstraZeneca paid $425 million to settle lawsuits alleging that patients suffered kidney injuries while using the heartburn drugs esomeprazole (Nexium) or omeprazole (Prilosec).
Case Studies
Here are two case studies about adverse effects tied to omeprazole:
When a young doctor began craving strange smells and salty flavors, he self-diagnosed it as a form of pica, but it took more than a year to find and fix the root cause of his condition. A drug taken long-term to treat GERD was the culprit.
Ask a Patient feature article: Strange Symptoms: Patient Case Study Involving Omeprazole
A man developed kidney issues after gastric bypass surgery/hiatal hernia surgery. This was found to be linked to the PPI (omeprazole) that was given as a prophylactic to PREVENT post-surgery ulcers. The patient developed high creatine levels and serious kidney injury during the weeks after surgery. The author concludes that prophylactic use of PPIs must be carefully considered and may not be worth the risk.
Recent case study in Cureus (medical journal): A Case-of-omeprazole-associated-acute-interstitial-nephritisTips about Meds for GERD and heartburn
Remember the Zantac (ranitidine) recalls that started in 2019 and led to the eventual discontinuation of all ranitidine products in the U.S. due to NDMA (cancer causing carcinogen) impurities? In November 2025, after a pause of more than five years, the FDA approved a reformulated version of H2 blocker ranitidine that addresses the shelf-life deficiencies and impurities present in the previous versions. It is not known if the previous “Zantac” will be relaunched with ranitidine. Zantac 360 is available but it contains famotidine as its active ingredient.
https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-reformulated-ranitidine-following-comprehensive-safety-reviewThere are many options for heartburn relief. Check out the three categories described at our website:
Antacid, H2 blocker, or PPI? Heartburn treatments in your kitchen, medicine cabinet, or at the drug store
H2 blocker, antacid, or PPI? Heartburn treatments in your kitchen, medicine cabinet, or drug store Did you miss the last edition? Check it out here: