January 2026: Recalls for Oxycodone, Wegovy, Rosuvastatin, Clearlife Nasal Spray; Blood Thinner Bleeding Antidote Withdrawn; Depo Provera, Addyi, and Hair Loss Drug News, More
New Safety Warnings for Opioid Medications
More than 60 opioid-containing medications have new safety warnings that were posted on the FDA website last week, and additional products are expected to be updated in the coming days.
In July 2025, the FDA ordered major safety updates to the labels for all opioid medications, including Schedule II pain relievers like Demerol, Fentanyl, OxyContin, MS Contin, and Nucynta, as well as several medications used to treat substance addiction like Suboxone, Subutex, Zubsolv, Naltrexone, and Vivitrol.
The updated labels give clearer warnings about serious long‑term risks, including a newly highlighted problem called “opioid‑induced esophageal dysfunction” (OIED), or a problem with swallowing and heartburn symptoms. Labels now also include information from new clinical trials, warn about drug interactions with gabapentin and other medications that affect the central nervous system, and advise healthcare providers to share information about the availability of overdose reversal agents like naloxone (Narcan).
Examples of updated labels: hydromorphone hydrochloride, demerol, fentanyl citrate, morphine sulfate, oxycontin, suboxone Find the complete list in the FDA Safety-Related Label Changes Database received December 22, 2025 and posted between January 5 -8, 2026.
SpecGx Oxycodone Recall
SpecGx (a division of Mallinckrodt) is recalling more than 350,000 bottles of opioid pain drug oxycodone-acetaminophen combination tablets due to a manufacturing defect. The imprint information that identifies a pill’s strength and type, which should always be stamped on the side of a tablet, may be missing or only partially visible. The recall affects tablets with 10 mg or 7.5 mg of oxycodone and 325 mg of acetaminophen with these lot numbers: 0523J23904, expires: 05/2027; 0523J24426, 0523J24427, expires: 06/2027; 0522J23493, expires: 03/2027. The recall is directed at retail pharmacies.
FDA Recall announcement
MediNatura Homeopathic Nasal Spray Recall Expands
Medinatura expanded its December 10 recall to include ALL Reboost brand homeopathic nasal spray products along with all Clearlife Allergy nasal spray products due to yeast/mold and microbial contamination. Both products are in 20 ml bottles. ReBoost and ClearLife were distributed nationwide via retail and internet sales(medinatura.com). As of January 10, these products are still available for sale at some online retailers. Customers should immediately discontinue using the nasal sprays.
Customers who purchased the product directly from MediNatura New Mexico, Inc. should contact MediNatura New Mexico to arrange for a refund. Consumers who purchased the product at other retailers should return it to the place of purchase.
FDA Recall Announcement
Wegovy Recall
Novo Nordisk is recalling four lots of weight loss drug Wegovy (semaglutide) injection pens because hair was found in a prefilled syringe. These were manufactured at Novo Nordisk’s Bagsvaerd, Denmark, facility. Lot numbers include RZFYK06, RZFYA53 (.5 mg with expiration of 3/2027); RZFHD52, RZFHW93 (1 mg with expiration of 10/31/2026).
FDA Wegovy Recall
Rosuvastatin Recall
AvKARE announced that 7,991 cartons of 10 mg rosuvastatin tablets, packaged in 50-tablet blister packs, are being recalled because of failed dissolution specifications, meaning they don’t dissolve as expected. The lot number is Lot #49124, with an expiration date of 12/31/2026. Rosuvastatin is a widely used cholesterol-lowering medication. This recall is directed at suppliers.
FDA Rosuvastatin Recall
Depo-Provera (medroxyprogesterone acetate) Depo-Subq Provera: Warning about Meningiomas
Pfizer updated contraceptive Depo-Provera and Depo-Subq Provera labels to warn that cases of meningiomas (a type of brain tumor that is slow growing and is usually benign) have been reported, primarily with long-term use. Patients should be monitored for signs and symptoms of meningioma. Depo-Provera (injected intramuscularly) was approved in 1992 and the subcutaneous version was approved in 2004. Both formulations are taken every three months (13 weeks).
These medications are not recommended as a long-term (i.e., longer than 2 years) birth control method unless other options are considered inadequate. However, many patients on the AskaPatient site report having taken it for well over 10 years and some report taking it for as long as 20 years.
A year ago, in January 2025, we reported that research has shown links between the injectable contraceptive Depo-Provera injections and an increased risk of developing meningiomas, which are usually benign brain tumors. The European Medicines Agency also required updates to labels.
Meningioma is the top reported adverse event for Depo-Provera with 1,306 cases reported (out of 13,287) in the FDA Adverse Events Database as of September 30, 2025.
Depo-Provera FDA Safety Label Update
Drug for Female Low Libido, Addyi, Expanded Approval for Postmenopausal Women
The FDA expanded its approval of Sprout Pharmaceutical’s Addyi (flibanserin) for the treatment of hypoactive sexual desire disorder (HSDD) in postmenopausal women under the age of 65. It was originally approved in 2015 for the treatment of HSDD in premenopausal women only. The label was updated with safety information from the clinical trials for postmenopausal women. Adverse reactions identified since its approval were added to the label: vomiting, asthenia (weakness), feeling abnormal, feeling drunk, malaise headache, faintness, gait disturbance, blurry vision, brain fog.
Addyi FDA Safety Update
Addyi has faced scrutiny over its safety and effectiveness. It has a long list of potential alcohol and drug interactions that may increase the risk of adverse reactions, including dizziness, low blood pressure, and drowsiness or sedation. Read more at
PharmedOut: “Is low libido a disease?” and MedShadow: “What Is Addyi? The Controversy Behind the Drug Nicknamed the ‘Female Viagra’”
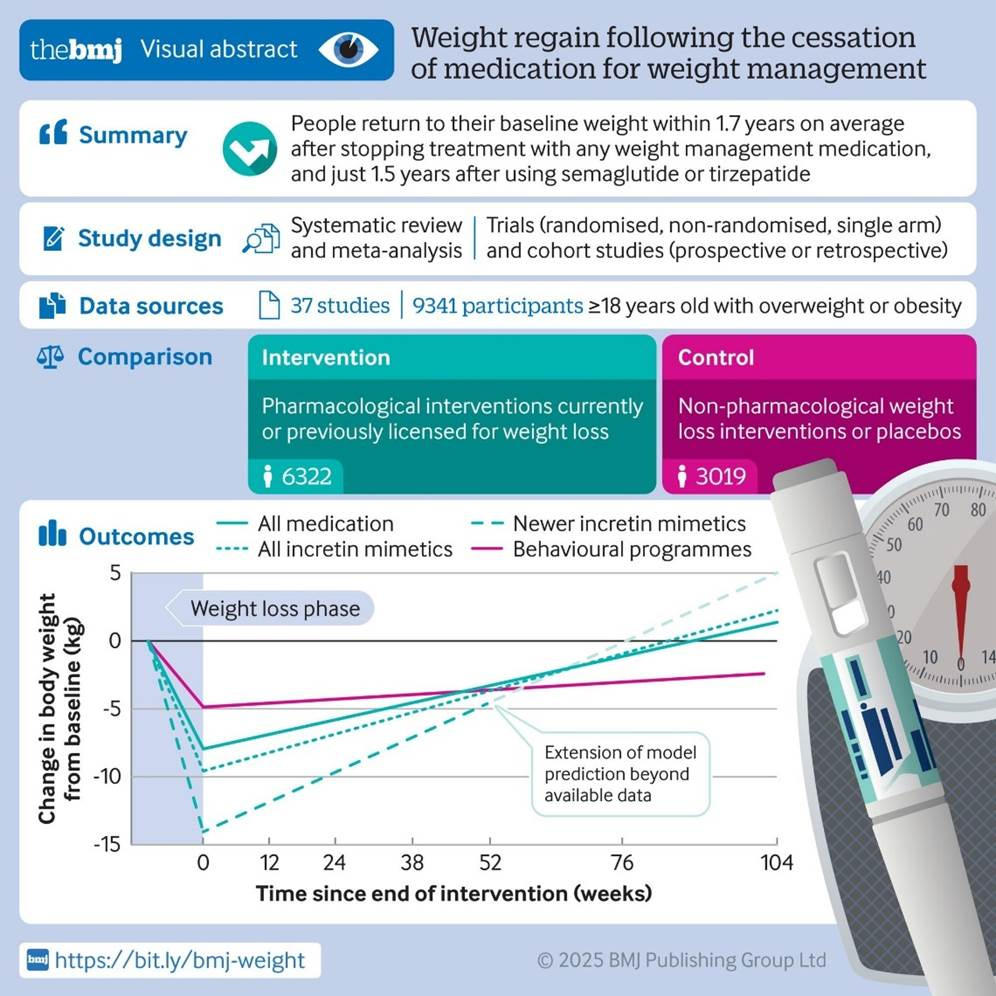
Research: Stopping GLP-1 Weight Loss Drugs Leads to Faster Weight Regain than Discontinuing Behavioral Weight Loss Program
Researchers at Oxford University reviewed 37 studies tracking more than 9,000 adults who stopped taking weight management medicines after being on them for an average of 39 weeks of treatment. Participants were followed for an average of 32 weeks after stopping. The analysis included older weight loss meds like orlistat (Xenical or Alli) and newer GLP-1 receptor agonists like semaglutide (Ozempic or Wegovy) and tirzepatide (Mounjaro or Zepbound).
Weight regain after stopping drugs happened more quickly (within 1.4 years) than after ending behavioral weight loss programs such as diet and exercise support. The authors suggest that even after a formal behavioral weight loss program ends, “the experience provides people with practical coping skills that they can continue to implement past the end of the intervention to help with weight loss maintenance.”
In the chart below, “all drugs” (solid green line) include semaglutide, tirzepatide, liraglutide, exenatide, cagrilintide, orlistat, phentermine+topiramate, lorcaserin, naltrexone+bupropion, sibutramine, rimonabant, phentermine, topiramate, benzphetamine, diethylpropion hydrochloride, phendimetrazine, fenfluramine, and dexfenfluramine.
“Newer incretin mimetics” include GLP-1s semaglutide and tirzepatide. The pink solid line indicates a non-drug weight loss intervention program.
Journal Article: The BMJ
Oxford University Press Release: “Weight Gain After Stopping Weight Loss Drugs Review”
New Warnings for Rheumatoid Arthritis, Plaque Psoriasis, and Thyroid Eye Disease (TED) Treatments
Learn about the new safety information recently added to the drug labels for Actemra, Gazvya, Kineret, Rystiggo, Stelara, and Tepezza.
MedShadow: New Side Effects for Autoimmune Drugs
Drug Research: New Hair Loss Treatment May be Safer and More Effective than Finasteride or Minoxidil
An acne treatment ingredient is being studied as a treatment for male pattern baldness. Cosmo Pharmaceuticals says results from two trials suggest its topical treatment clascoterone 5% solution could become a game-changing treatment for male-pattern hair loss. The studies included 1,465 participants with male androgenetic alopecia (AGA) in 50 sites across Europe and the U.S. One trial showed a 539% relative improvement in target-area hair count (TAHC) through six months, while the other trial showed a 168% gain versus placebo. The difference in the trial figures is driven “entirely by baseline hair counts, not by a difference in drug performance,” said Cosmo CEO Giovanni Di Napoli.
Clascoterone 1% cream (brand name Winlevi) was approved in 2020 as a treatment for acne in patients age 12 and up. As a hair loss treatment, the topical androgen receptor inhibitor works by blocking dihydrotestosterone (DHT) directly at the hair-follicle receptor without systemic absorption, targeting the root cause of male-pattern hair loss without the risks of oral therapies. According to Cosmo, of 166 million men in the U.S., 65 million have AGA, including 16 million who have been treated and 27 million who are seeking treatment.
Fierce Pharma: Cosmo Shares Zoom on Results for Male Pattern Baldness Study
Andexxa Withdrawn from the Market
The FDA announced the withdrawal of Alexion’s Andexxa (Andexanet ALFA) from the market due to safety concerns. After being granted “accelerated” approval in 2018, continued approval was contingent on final clinical trials data and monitoring of adverse event reports. Andexxa was used as an antidote, on an emergency basis, to help curb severe bleeding in patients taking the blood thinner rivaroxaban (Xarelto) or apixaban (Eliquis). If a patient needs emergency surgery, with life-threatening or uncontrolled bleeding, an antidote is sometimes needed to help stop the bleeding. Unfortunately, unfavorable postmarketing safety data on thromboembolic events, including serious and fatal outcomes, have led the FDA to determine that the risks of Andexxa outweigh its benefits.
FDA Safety Communication: “Update on the Safety of Andexxa”
What will doctors now use to treat uncontrolled bleeding emergencies in patients taking oral blood thinners? Another medication, Praxbind, is approved as antidote for uncontrolled bleeding in patients taking the blood thinner Pradaxa. Unfortunately, like Andexxa, Praxbind also comes with a risk of thromboembolic events (like stroke), and it can only be used as an antidote for Pradaxa. Patients taking warfarin as a blood thinner are often given Vitamin K as an antidote. For Xarelto and Eliquis patients, there are some off-label or older I.V. options left.
Medication Tips: Blood Thinners
Check out the People’s Pharmacy’s advice about the bleeding risks associated with oral anticoagulants (oral blood thinners like Eliquis, Xarelto, and Pradaxa):
“Patients Walk a Dangerous Tightrope on Blood Thinners”
Read or add patient experiences for Coumadin, Warfarin, Xarelto, Eliquis, Lovenox, or Pradaxa blood thinners/oral anticoagulants at our website.
Did you miss our last newsletter? Read it here:
Here is one of our most-read newsletters in 2025:
Thank you for reading this edition of Ask a Patient® Health News: Drugs & Treatments! Visit our website for more information about medications, including patient reviews and ratings for prescription drugs, along with patient experiences with common vaccines and some alternative treatments.





How long will it take for Wegovy Pill version Recall ?
https://geoffpain.substack.com/p/wegovy-pill-pushers-go-berserk-with