July 23, 2023: Rx-OTC mini-pill approved; new leukemia drug; RSV preventive med for babies; psychedelics as meds; stakeholders weigh in; safety of new heart drug; thyroid drug side effects
New Drug Approvals
Perrigo's Opill (norgestrel) was approved by the U.S. Food & Drug Administration (FDA) as the first daily oral birth control pill to be available in the U.S. without a prescription. While officially an "Rx-to-Otc Switch," Opill's application is actually based on a discontinued prescription mini-pill (progestin only, no estrogen) called Ovrette, which was removed from the market (not for safety or efficacy reasons) by Wyeth in the early 2005. Pfizer acquired Wyeth in 2009 and in 2015 sold the rights to Ovrette to French company HRA Pharma, owned by Perrigo. HRA Pharma then changed the name of the product to Opill. Perrigo also makes Ella, (ulipristal acetate) emergency contraception pill.
The FDA notes that Opill must be taken at the same time every day and should not be used by those who have or have ever had breast cancer. This drug will not require a pharmacist consultation, and can be sold by any store including grocery stores, convenience stores, and online.
FDA Press Announcement
FDA Safety Announcement
More on the Opill from KFF Health News:
https://kffhealthnews.org/news/article/over-the-counter-birth-control-pill-cost-insurance-coverage-opill-perrigo/
Opill has no age restriction for product purchase. It will be available as a one, two, three, or six month supply.
https://www.al.com/news/2023/07/first-over-the-counter-birth-control-pill-approved-by-fda-opill-will-have-no-age-restriction.html
In the Ask a Patient® opinion poll about whether the oral contraceptive should be approved for over-the-counter purchase, 57% said the pill should be available OTC, while 27% thought it should be available OTC but require a pharmacist consultation. 10% of respondents thought it should NOT be available over-the counter, and 5% weren't sure.
Vote here for another week: https://www.askapatient.com/resources/healthcarepoll.asp
In many Asian, African, and South American countries the pill is available without a prescription or with only a pharmacist consultation, but in European countries (except U.K.), Australia, Japan, and others, a prescription is still required. (see map in the May 14 issue of Ask a Patient Health News).
AstraZeneca's Beyfortus (nirsevimab-alip) intramuscular injection was approved by the FDA for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in infants born during or entering their first RSV season, and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. This monoclonal antibody treatment contains laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses. Beyfortus comes with warnings and precautions about serious hypersensitivity reactions, including anaphylaxis.
https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
Daichi Sankyo's Vanflyta (Quizartinib) was approved by the FDA to treat newly diagnosed adult patients suffering from an aggressive type of blood cancer, acute myeloid leukemia (AML) that is FLT3-ITD positive. It is to be taken along with chemotherapy and as maintenance therapy after chemotherapy. Quizartinib is available through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). The drug has a boxed warning for the risk of cardiac arrest and QT prolongation, a heart signaling disorder.
Business Wire
https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-quizartinib-newly-diagnosed-acute-myeloid-leukemia
Heart Drug Safety Concerns
In May 2023, FDA approved Lexicon's Inpefa (sotagliflozin), the first SGLT1/SGLT2 drug to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure in adults with heart failure condition or with Type 2 diabetes, chronic kidney disease, and other cardiovascular risk factors. But the road to approval was bumpy: it had been rejected by the FDA twice before, under a different brand name. Previous co-sponsor Sanofi withdrew from the partnership after mixed trial results of sotagliflozin as a treatment for Type 2. The European Union, having approved it for Type1 diabetes under the name Zynquista, withdrew it from the market at the request of the company. Most recently, the serious risk of diabetic ketoacidosis was a major concern for the divided FDA advisory committee.
With so many red flags and changes in proposed indications for sotagliflozin, one wonders: is the drug safe? Check out health writer Emma Yasinsky's account of the "long and winding road" leading to sotagliflozin's approval by the FDA. And if your doctor suggests that you take it, make sure to ask two essential questions.
https://medshadow.org/heart-failure-drug-for-diabetes-fda/
Taking Ozempic or another GLP-1 Drug and Planning Surgery?
Because GLP-1 agonist drugs for diabetes or weight loss like Ozempic or Wegovy keep food in the stomach longer, the American Society of Anesthesiologists is recommending that people on a GLP-1 agonist like Ozempic stop taking it prior to surgery. "That food left in the stomach increases the risk you will vomit while under anesthesia," said ASA President Dr. Michael Champeau. If you take such a drug once a day, you should not take your daily dose the morning of surgery; if you take the drug once a week, you should stop it at least a week in advance. Other GLP-1 diabetes drugs include dulaglutide (Trulicity), exenatide (Byetta), liraglutide (Victoza) and lixisenatide (Adlyxin), and Rybelsus.
https://www.upi.com/Health_News/2023/07/03/Ozempic-Wegovy-anesthesia-warning/9831688410910/
Psychedelics as Medications
Effective July 1, Australia's drug regulator Therapeutic Goods Administration designated MDMA (a psychedelic drug also known the party drug "molly" or "ecstasy") as a controlled substance rather than an illegal substance. Earlier this year (as we reported in April 2023) it was approved in Australia as a treatment for post-traumatic stress disorder (PTSD). The world's drug regulators are closely watching what happens in Australia, the only country to legalize MDMA. Psychologist Mark Travers describes treatment potential and interviews experts about research progress with three psychedelic drugs (MDMA, Psilocybin, and Ketamine). "We've got no data on long-term outcomes at all, so that worries me a lot, which is one of the reasons why I'm doing my very large study,” Professor Susan Rossell of Swinburne University explained in an interview.
https://www.forbes.com/sites/traversmark/2023/07/03/mdma-psilocybin-and-ketamine-3-psychedelics-with-different-mental-health-use-cases/?sh=74a7a5a12e38
To support the research of psychedelic drugs as mental health treatments in the U.S., the FDA posted an 11 page document, "Psychedelic Drugs: Considerations for Clinical Investigations; Draft Guidance for Industry" and asked for public comment. The FDA notes that its use of the term "psychedelic" in the document includes classic psychedelics, typically understood to be 5-HT2 agonists such as psilocybin and lysergic acid diethylamide (LSD), as well as entactogens or empathogens such as methylenedioxymethamphetamine.
Since the posting on June 26, 41 comments from the public have been received. So far, comments have mainly come from individuals, including therapists, researchers, nurses, patients, and anonymous contributors. Some applaud potential approval of the drugs, while others say that any new treatments should be an adjunct to therapy, not a treatment in and of itself. For example, one commenter who works in clinical trials of psychedelics, says, "the bias in drug use in the United States is to ask the drug to do all of the work and that is a poor model for the use of drugs that are primarily psychological in nature and are oriented to use for mental health outcomes. The best use of antidepressants has been shown to be in addition to psychotherapy. It will be the same with psychedelics."
The Neurosensory & Neuroregenerative Research Foundation (NRF) expressed concern about the danger of Hallucinogen Persisting Perception Disorder (HPPD). HPPD are visual oddities that can appear long after the effects of psychedelics wear off. They say "it’s looking more like a gold rush to legalize psychedelics (as was the case with cannabis) than patient care."
Read the FDA guidance document and comments; add your comments until August 25.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/psychedelic-drugs-considerations-clinical-investigations
Comments: https://www.regulations.gov/docket/FDA-2023-D-1987/comments

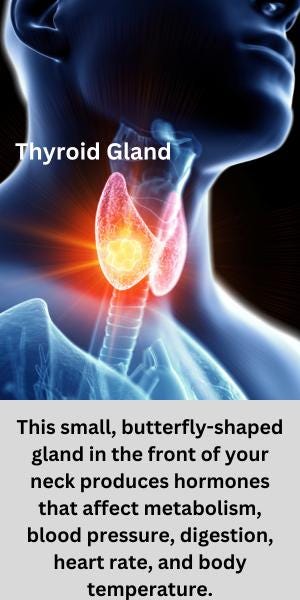
Medications for Underactive Thyroid (Hypothyroidism)
The thyroid is a small, butterfly-shaped gland in the front of your neck. It makes hormones that control the way your body uses energy, affecting breathing, heart rate, metabolism, blood pressure, digestion, and body temperature. When you don't have enough of the thyroid hormone, the condition is called hypothyroidism. Levothyroxine (Synthroid brand name), a synthetic form of the hormone, is often prescribed as treatment. According to the 2022 Health Distributor's Association Factbook, levothyroxine is one of the top five most-prescribed generic drugs in the U.S., with 83.4 million prescriptions filled in 2021.
Winnie Yu, coauthor of The Everything Guide to Thyroid Disease, describes her own recent diagnosis and experiences with Synthroid medication. She says that "pinpointing the correct dosage is key."
https://medshadow.org/synthroid-side-effects/
In addition, check out Medshadow's comprehensive guide to thyroid conditions. Learn about types of conditions, associated symptoms, treatments, and side effects.
https://medshadow.org/thyroid-problems/
Ask a Patient reviewers encountered the following side effects while taking Synthroid. (Note that some of the side effects align with symptoms of the condition itself.) Click to read examples of patient mentions of the side effect.
Osteoporosis*
*These side effects can occur over the long term when taking Synthroid, and are consistent with having too much thyroid hormone or hyperthyroid.
Some patients find success with Armour Thyroid, a natural thyroid product usually made from a pig's thyroid gland.
https://www.askapatient.com/viewrating.asp?drug=99900002&name=ARMOUR+THYROID
More thyroid drugs and related reviews (including Levoxyl, Unithroid, Cytomel, Tirosent) are listed at Ask a Patient.
To learn what other drugs can interact with levothyroxine or what other medications can otherwise affect thyroid function, check out "Meds that Mess with your Thyroid."
https://medshadow.org/meds-that-mess-with-your-thyroid/
Medication Tip: the right dose of melatonin
Melatonin supplements are available in a wide range of dosages, from 1 milligram up to 10 milligrams, and can be taken in pill form, as dissolvable tablets, liquid drops, or even gummies. However you take it, experts say the best time to use melatonin is about 30 minutes to an hour before bedtime. The proper dosage varies depending on age, why you’re taking it, and medical history. Read expert advice and safety information about melatonin supplements. This guide also includes a "Melatonin Dosage Chart by Age Group."
https://www.singlecare.com/blog/melatonin-dosage/
Visit us as AskaPatient.com for drug ratings and reviews provided by patients along with more news and health information. Note to readers: Ask a Patient® Health News (regular edition) will be sent on Tuesday, July 25. If you are not already subscribed, sign up today.
If you like what you are reading, hit the ❤ (Like) button below or at the top so more people can discover this newsletter on Substack 🙏 (Thank you!)