World’s Essential Medicines: What did and didn't make the list; Medical device sterilization dilemma; Eye drops for nearsightedness, Meds for liver, lung disease, fibromyalgia; ❤️for beet juice
Notable Updates to “The Selection and Use of Essential Medicines 2025”
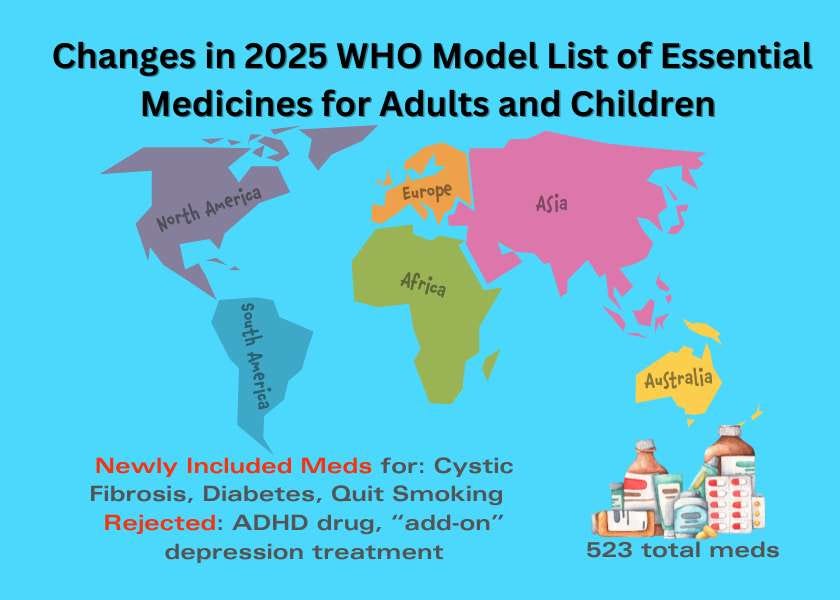
The World Health Organization’s 24th edition of its “Model List of Essential Medicines” (EML) and 10th edition of the “Model List of Essential Medicines for Children” (EMLc) were released September 5 and include 523 medicines for adults and 374 pediatric medications. Updated every two years and used by more than 150 countries, the EML serves as a catalog of the drugs that WHO believes should be available to meet a country’s priority health care needs. Inclusion of a drug on the list helps to ensure access to vital medications for people in poorer countries.
The WHO Essential Medications Expert Committee met in Geneva in May 2025 and after considering 59 applications, selected 20 new drugs for inclusion in the EML and 15 new drugs for the EMLc. Notable additions:
Semaglutide, a GLP-1 drug, was added to treat patients with Type 2 diabetes with established cardiovascular disease (CVD) or chronic kidney disease (CKD); and with obesity (body mass Index (BMI) ≥ 30kg/m2). Semaglutide is the active ingredient in Ozempic and Wegovy. The Committee recommended that WHO work on existing approaches for managing prices and evaluate alternative strategies to improve affordability and access to reduce the global burden of diabetes. Semaglutide is expected to go off-patent in 2026 in the U.S. (although it varies by country), so more affordable biosimilar (generics) may be available soon.
Pembrolizumab (Keytruda brand name) was added to the EML as a first-line therapy for metastatic cervical cancer, metastatic colorectal cancer, and metastatic non-small cell lung cancer. For metastatic non-small cell lung cancer (NSCLC), atezolizumab and cemiplimab were included as therapeutic alternatives.
The combination of elexacaftor + tezacaftor + ivacaftor (Trikafta brand name) was added as a treatment for cystic fibrosis, along with ivacaftor (Kalydeco brand name) as a stand-alone treatment.
Cytisine was added as a nicotine addiction (stop-smoking) treatment. Cytisine is not approved yet in the U.S., but has an application under consideration.
The following drugs were rejected from inclusion in this edition:
Semaglutide was NOT included as a stand-alone treatment for obesity. (However it was included for Type 2 diabetes with obesity as noted above.)
The stimulant methylphenidate (brand name Ritalin) was NOT included as a treatment for ADHD for adults or children. The committee had concerns about long-term safety of the medication. They also noted that the drug is already included on some countries' list of essential medications. Attention deficit hyperactivity disorder (ADHD) is not a medical condition included in the EML or the EMLc.
Brexpiprazole (Rexulti brand name) was NOT included as an add-on treatment for major depressive disorder in adults based on evidence from short-term studies showing only modest incremental benefits compared to placebo, with the committee noting other atypical antipsychotics and lithium had similar or superior gains.
The EMLc continues to exclude drugs treating mental and behavioral disorders in children.
Beginning in 2023, the WHO removed the entire section 24, which covers Mental and Behavioral Disorders, from the Children’s Essential Medications List (EMLc). No mental and behavioral disorder medications are recommended by WHO for patients under the age of 13.
Press Release: "WHO updates list of essential medicines to include key cancer, diabetes treatment"
WHO Model List of Essential Medicines, 24th Edition (2025) Executive Summary
WHO 10th List of Essential Medicines for Children (2025)
The Mental and Behavioral Disorders section of the EML includes medications deemed essential for treating depression, bipolar disorders, anxiety disorders, obsessive compulsive disorders, psychotic disorders, and substance abuse in adults only. Check out our summary of the included drugs.
Commercial Medical Sterilization Facilities Granted Extra Time to Comply with New EPA Standard
Susan Kamuda developed breast cancer after decades of exposure to ethylene oxide (EtO) emissions from Sterigenics’ commercial medical device sterilization plant near her home in Willowbrook, Illinois. Her 2022 lawsuit (in which she was awarded over $300 million by a Cook County jury), followed by hundreds of other such lawsuits, brought nationwide attention to the cancer risks of EtO facility emissions.
Ethylene oxide (EtO) gas is essential for sterilizing many heat- and moisture-sensitive medical devices, such as catheters, stents, wound dressings, and implants. These are items that often have hard-to-reach components and cannot be sterilized using other available methods like autoclave steam pressure chambers. The FDA says that ethylene oxide is used to sterilize 20 billion devices sold in the U.S. every year, accounting for about 50 percent of devices that require sterilization. In August 2022, the FDA announced efforts to find alternatives to ethylene oxide sterilization methods and since then has held multiple town halls on the topic. In January 2024, they announced a plan to broaden the use of vaporized hydrogen peroxide (VH2O2), an established method of sterilization for medical devices.
New EPA regulations require commercial sterilization facilities to drastically reduce EtO emissions. Deadlines were set for 2025 for reducing emissions or finding alternative ways to sterilize the equipment, but there have been technological and financial hurdles, leading to delayed compliance.
The White House recently granted some sterilization plants regulatory relief, giving them more time or flexibility to meet standards while maintaining critical sterilization capacity. Many communities surrounding the facilities are not happy that the facilities will be allowed to continue to operate without fixing the emissions. Check here for a list of the facilities:
White House: List of Medical Sterilization Facilities Granted Regulatory Relief
33 states have at least one commercial EtO commercial sterilization facility. The facility names and addresses in the link below are listed by state and city.
Environmental Protection Agency: EPA List of Commercial Sterilization Facilities
Hazardous Air Pollutants: Ethylene Oxide
New Drug Approvals
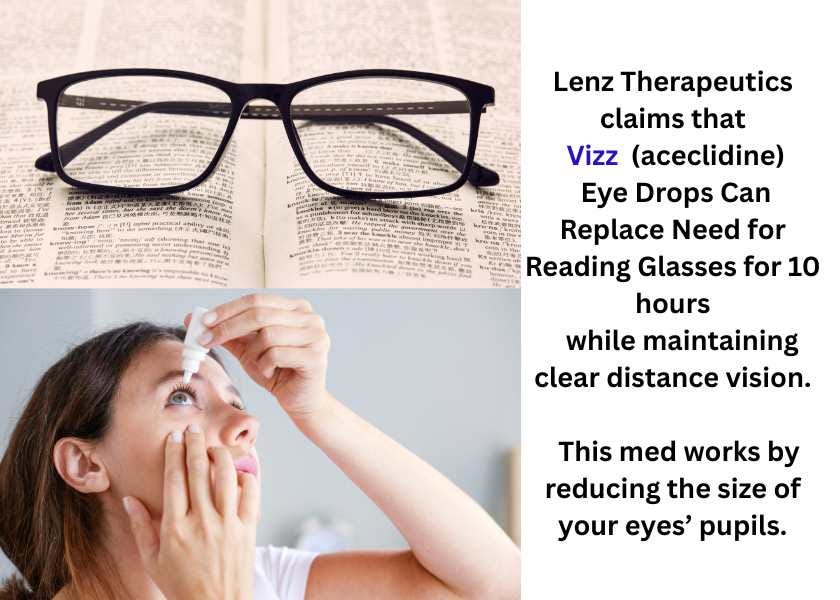
Vizz (aceclidine) Eye Drops for Presbyopia
LENZ Therapeutics’ Vizz (aceclidine) was approved for the treatment of age-related fuzzy near vision (presbyopia) in adults. In its press release, LENZ states that acedeline contracts the sphincter of the iris, “resulting in a pinhole effect that extends depth of focus to improve near vision without causing a myopic shift.” This allows for clear distance vision, and the effects are said to last for about 10 hours. If needed, contact lenses for distance vision may be used after inserting the Vizz eye drops. While aceclidine has never been approved for use in the U.S. until now, Ophthalmology Times reports that it in Europe it is a glaucoma treatment.
Allergan’s Vuity, approved in 2022 and Orasis’ Qlosi, approved in 2024, also correct presbyopia by constricting the pupils, but they contain the active ingredient pilocarpine and require additional doses for the longer-term (9 hours) effect. All three of these products warn users to be cautious driving at night or when doing hazardous activities in areas with dim light. Ophthalmology Times reports that two additional drugs for correcting presbyopia are in the FDA pipeline.
LENZ Therapeutics Press Release
Ophthalmology Times: “The emerging era of presbyopia-correcting eye drops: What’s next?”
Wegovy (semaglutide) Subcutaneous Injection for Liver Disease
Novo Nordisk’s Wegovy (semaglutide) received FDA approval for treating adults with the liver disease noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH), with moderate to advanced liver scarring. MASH is a severe form of metabolic-associated fatty liver disease that develops when fat buildup in the liver causes inflammation and scarring. The FDA states that approximately 6% of U.S. adults (14.9 million people) have MASH. Wegovy was originally approved in 2017 for weight loss in patients with obesity.
FDA Press Release
Tonmya (cyclobenzaprine hydrochloride) Sublingual Tablets for Fibromyalgia
Tonix Pharmaceuticals’ Tonmya (cyclobenzaprine hydrochloride sublingual tablet) was approved for the treatment of fibromyalgia in adults. Until now, the FDA has approved only three medications for fibromyalgia: duloxetine (Cymbalta), pregabalin (Lyrica), and milnacipran (Savella). Pain News Network cautions that this is not a breakthrough treatment: Tonmya acts more like a sleeping pill than a pain drug. Cyclobenzaprine hydrochloride has been available since 1977 to treat muscle spasms. The FDA is requiring that Tonix conduct additional clinical trials to assess adverse maternal, fetal, or infant outcomes.
Fibromyalgia is a chronic condition that causes pain all over the body, fatigue, and other symptoms. Read more about fibromyalgia at Medline Plus.
Pain News Network: “FDA Approves First New Fibromyalgia Drug in 15 Years”
FDA Approval Letter
Brinsupri (brensocatib) Tablets for Bronchiectasis
Insmed's Brinsupri (brensocatib) was approved for patients age 12 and up to treat non-cystic fibrosis bronchiectasis, a chronic condition that damages the lungs and makes it hard to clear mucus. According to Insmed, there are about 500,000 people in the U.S. diagnosed with NCFB, with millions more estimated to be living with the disease globally. Unlike other respiratory diseases that are characterized by airway narrowing, bronchiectasis causes airways to permanently widen, making it harder to clear mucus and bacteria, leading to persistent inflammation and infection. Brinsupri tablets are taken once daily.
Insmed Press Release
Beetroot juice for lowering blood pressure
The People’s Pharmacy’s Joe Graedon revisits the topic of beet juice as a good dietary choice for lowering blood pressure, explaining new research that supports its use. According to the study, here’s how it worked: the beet juice led to healthy changes in the oral microbiome, which helped metabolize the nitrates in beet juice into nitric oxide, leading to more relaxed blood vessels.
People’s Pharmacy: “Beet Juice was Better than Drugs for Lowering Blood Pressure”
Did you miss these recent posts?
Companies marketing naturally-derived thyroid hormone for hypothyroidism received warnings from the FDA. The biological medications aren’t FDA-approved, but are helpful for some patients for whom synthetic versions like levothyroxine just don’t work.
Next edition will include Drug Safety Updates for September. Thank you for reading! Visit our website at www.askapatient.com for more on drug treatments.