Unique Glasses for Kids; Drugs for Common Diseases Get Fast-Tracked; Cancer and Pain Treatment Research; Gabapentin Update; Flu Season News, Protein Supplements Tips; Bankrupt Rite Aid Sues CVS
This week we're featuring treatments approvals, drug research, pharmacy news, and updates on flu shots. Plus a round-up of three safety investigations of protein powders
New Drugs & Treatments
Glasses for Preventing Progression of Nearsightedness (Myopia) in Children
Since the 2010s, contact lenses designed to slow the development of nearsightedness (myopia) in children have been available in the U.S., but many kids have trouble inserting and wearing contact lenses. Now, there is an eye glasses option. The FDA authorized Essilor’s Stellest eyeglass lenses to correct and slow the progression of myopia, with or without astigmatism in children 6 to 12 years old at the start of treatment. Myopia affects approximately 40% of the U.S. population, with prevalence increasing rapidly among children and adolescents. The Essilor Stellest eyeglass lenses have a clear 9mm diameter area in the center, which is surrounded by rings of tiny, raised dots (peripheral “lenslets”). The tiny, raised dots provide peripheral light defocus, which may help to slow the progression of myopia in children. It is recommended that the child wears the lenses for a minimum of 10 hours per day for at least 6 days per week.
FDA Press Release: “FDA Authorizes Marketing of First Eyeglass Lenses to Slow Progression of Pediatric Myopia”

Keytruda Subcutaneous Formulation
The FDA approved Merck’s subcutaneous (under-the-skin) version of cancer drug Keytruda, offering an alternative to drug infusion but without meaningful differences in outcomes or safety. The new formulation also allows Merck to retain additional years of patent protection for Keytruda.
According to Bloomberg Law, “Merck is performing a sleight-of-hand known as a “product hop” by tweaking how the drug is delivered. Last year, Keytruda brought in about $29.5 billion globally for Merck. In February 2023, four members of Congress urged the US Patent and Trademark Office to scrutinize Merck’s Keytruda patent portfolio, saying its applications appeared to reflect “anti-competitive business practices,” such as “patent thicketing” and product hopping.
FDA Press Release: FDA Approves pembrolizumab-and-berahyaluronidase-alfa-pmph subcutaneous injection
Bloomberg Law: “Merck’s New Keytruda Shot is a Rare Real Time Product Hop”
Have you taken Keytruda? View ratings or add your experience.
New Treatment for Barth Syndrome
The FDA granted accelerated approval to Stealth Biotherapeutics’ Forzinity (elamipretide) subcutaneous injection as the first treatment for Barth syndrome, in patients weighing at least 30 kg (66 pounds). As a condition of accelerated approval, FDA is requiring a post-approval randomized, double-blind, placebo-controlled trial to confirm that the changes seen on knee muscle strength translate into patient benefit. Barth syndrome is a rare, serious and life-threatening disease of the mitochondria (the energy-producing parts of cells) that primarily affects boys. The disease begins with severe heart failure in infancy, and often causes premature death. Patients who survive into adolescence and adulthood often have fatigue, poor stamina, and exercise intolerance.
FDA Press Release: FDA Grants Approval for First Treatment for Barth Syndrome
Read more about Barth Syndrome at Medline Plus
Nine Drugs for Common Conditions Slated for New Fast Approval Program
In June, FDA Commissioner Martin Makary introduced a new drug approval pathway, called the National Priority Voucher (CNPV) pilot program. The CNPV pilot program aims to reduce drug and biological product application or efficacy supplement review times from about a year to just 1-2 months. This CNPV program is different than the “Orphan drug” or “Accelerated approval” pathway that speeds up drug approvals for rare diseases. Instead, it focuses on “accelerating cures and meaningful treatments with historic public health impact for Americans,” with a focus on common chronic conditions, high prevalence diseases, increased affordability, and on-shoring drug development and manufacturing so that there is less dependent on foreign facilities for production of essential medicines.
The first nine drugs selected to be reviewed under this program include:
Pergoveris for infertility
Teplizumab for Type I diabetes
Cytisinicline for nicotine vaping addiction
DB-OTO for deafness
Cenegermin-bkbj for blindness
RMC-6236 for pancreatic cancer
Bitopertin for porphyria
Ketamine for domestic manufacturing of a critical drug for general anesthesia
Augmentin XR for domestic manufacturing of a common antibiotic
FDA Press Release: “FDA Awards First-Ever National Priority Vouchers to Nine Sponsors “
Drug Research
Potential for RA Drug as New Diabetes Type 1 Treatment
Rheumatoid arthritis (RA) drug baricitinib (brand name Olumiant) has shown promise as a treatment for Type 1 diabetes. In 2023, an Australian trial reported that a daily pill of baricitinib could safely preserve the body’s own insulin production and slow the development of type 1 diabetes in those recently diagnosed. A follow-up trial presented at the Annual Meeting of the European Association for the Study of Diabetes, held in Vienna last month, revealed that once baricitinib treatment was stopped, participants’ diabetes progressed.
Newsweek: Daily Pill May Slow Progression of Type 1 Diabetes-‘Really Exciting Step’
Read or add patient reviews or view the adverse events case summary for Olumiant (baricitinib). Olumiant gained additional approval recently as treatment for severe alopecia (autoimmune-related) and for hospitalized patients with Covid-19 requiring supplemental oxygen.
Aspirin and Preventing Colon Cancer Study
A Swedish research study published last month in the New England Journal of Medicine concluded that aspirin can dramatically reduce the likelihood of colorectal cancer recurrence in patients with a certain gene mutation. Patients who had their tumors removed received either 160 mg of aspirin (half a standard tablet) or placebo per day and were followed for three years. These cancer patients had a common gene mutation called PIK3CA. There was a 7.7 percent recurrence rate in the aspirin takers compared to a 14 percent return in the patients on placebo, a relative risk reduction of 50 percent. The People’s Pharmacy’s Joe Graedon notes that a study also published in the New England Journal of Medicine 34 years ago concluded favorable results for colon cancer patients taking aspirin.
People’s Pharmacy: “Aspirin Vs. Cancer: What are We Waiting For?”
Pain Treatment Research
A Cleveland Clinic study found that a series of low-dose ketamine infusions can help reduce chronic pain and improve quality of life for many patients. Nearly half of those who had infusions reported significant improvements that lasted for months. The study was published in Regional Anesthesia & Pain Medicine.
Cleveland Clinic: “Cleveland Clinic Study Demonstrates Safety, Effectiveness of Ketamine for Chronic Pain”
Pharmacies in Jeopardy Over PBM Practices
The National Community Pharmacists Association reports that Rite Aid, once the third-largest pharmacy chain in the United States, has closed all of its stores and filed a lawsuit against CVS Caremark (one of the “big three” Pharmacy Benefit Managers), alleging that it charged Rite Aid $500 million in clawbacks for prescriptions that were already filled and paid for. It said those penalties violated their contract and lined the PBM’s pockets. Ultimately, they say CVS drove them out of business and to boot, CVS has now acquired about 717 stores’ worth of Rite Aid’s customers’ prescriptions.
The Federal Trade Commission (FTC) is investigating the big three PBMs for their business practices and allegations of anti-competitive behavior. Three major PBMs control more than 80 percent of all prescriptions filled in the U.S.: CVS Caremark, OptumRx and Express Scripts.
NCPA Press Release: “Rite Aid Sues CVS Caremark for Anticompetitive Behavior”
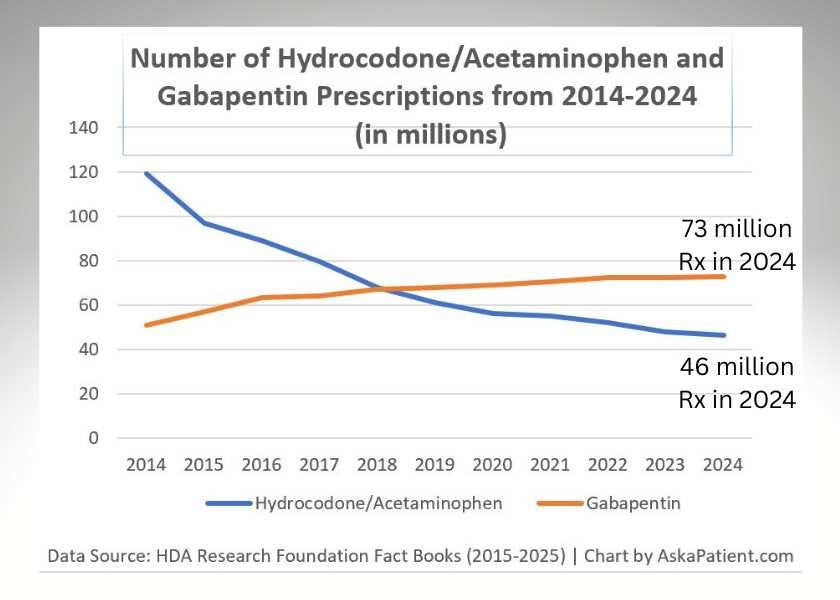
Gabapentin Prescriptions Soar
The number of gabapentin prescriptions dispensed last year increased to over 70 million; it is the fifth most prescribed drug in the U.S. (only behind atorvastatin, amlodipine, levothyroxine, and lisinopril). Our updated article explains some of the contributing factors to this trend and emerging safety concerns.
AskaPatient: “What is Gabapentin Used for and Why are So Many Patients Taking It?”
2025-2026 Influenza Season Update
Just like last flu season, each of the nine influenza vaccines offered this year are trivalent (cover three strains of flu virus) instead of the usual quadrivalent vaccines. The “Yamagata” flu strain, which had its heyday in the 1990s, has not been detected in the population since March 2020, thanks to the dramatic drop in flu infections during the Covid-19 pandemic. That strain was dropped from the vaccine last year.
However, even with less strains around, last year was a very bad flu season; in fact, the CDC says that the 2024-2025 influenza season was the first high-severity influenza season for all age groups since the 2017-2018 season. This season, the CDC predicts we will have “moderate” levels of influenza. Check out the list of vaccines (including a self-administered one and egg-free varieties) and more information about the upcoming flu season.
Ask a Patient: “2025-2026 Flu Season: Influenza Vaccine Supply”
Supplement Tips: Results of Three Labs Testing for Lead in Protein Powders
Are you getting enough protein? The recommended daily protein requirement is about 0.40 gram per pound of body weight for sedentary individuals, 0.55 to 0.65 gram per pound for endurance athletes and 0.65 to 0.80 gram per pound for strength athletes. Consumer Reports notes that for a 170-pound adult, that’s a daily intake of about 61 grams of protein, which can be achieved by eating a cup of plain Greek yogurt and 3.5 ounces of chicken breast.
So while most people should be able to get enough protein from their diet, there are research-backed medical reasons people might need more protein: to retain muscle mass during periods of bed rest, maintain muscle during chemotherapy, help with recovery after hospitalization, help control blood sugar levels, and to increase the protein level in the blood of kidney failure patients undergoing dialysis.
While protein powders and drinks can be healthy, testing has shown that high levels of lead and other heavy metals are present in some products. Consumer Reports, Consumer Labs, and the Clean Label Project recently conducted heavy metal tests (lead, mercury, cadmium, arsenic) on various protein powders, and all three found that plant-based products had far more lead than whey (dairy-based) protein powders and shakes.
Of the 23 protein powders and ready-to-drink shakes tested by Consumer Reports, a single serving contained as much as ten times more lead than CR’s food safety experts say is safe to consume in a day. One of Consumer Reports’ recommended products was Optimum Nutrition Gold Standard 100% Whey Chocolate, which was also a Consumer Labs’ Top Pick for flavored whey protein (Double Rich Chocolate version).
Consumer Labs also found that overall, the non-flavored products had less contaminants than flavored ones, and that on average, organic protein powders contained three times more lead and twice the amount of cadmium as non-organic products.
The news isn’t all bad; the Clean Label Project, which recently tested 160 top-selling protein powders, found that only 3 of the powders contained detectable bisphenols (including BPA and BPS).
Find the complete reports, with product recommendations based on test results:
Consumer Reports: “Protein Powders and Shakes Contain High Levels of Lead”
(free article)
Consumer Lab: “Protein Powders, Shakes, Drinks, Nutrition Drinks”
(subscription required)
Clean Label Project Protein Powder Testing Results: Clean Label Project Protein Study 2.0
(free report; also includes a link to their downloadable “Clean 16 protein powder products”
Thank you for reading! Next time, we’ll feature drug safety news, including product recalls, new risk statements added to drug labels, and more.
Did you miss the last edition of this newsletter? If so, you may read it here:




Great roundup of health news! The Keytruda subcutaneous formulation story is fasinating but troubling. Merck generating almost 30 billion from Keytruda and then doing product hopping to extend patent protection is exactly the kind of behavior that keeps drug prices artificially high. The fact that there are no meaningful differences in outcomes or safety makes it even more clear that this is about patent games, not patient benefit. Four members of Congress calling out the patent thicketing is a good sign that regulators are starting to pay atention to these tactics. Hopefully the FTC's scrutiny of PBMs mentioned in the Rite Aid story leads to real structural changes in how prescription drug markets operate.