Huge recall of Glaucoma and Allergy Eye Drops, Recall of Granules' Generic Adderall XR; Acetaminophen Safety; Semaglutide and Serious Eye Condition Warning; Recommended Videos and Podcast
Glaucoma and Allergy Eye Drops Recall by Apotex
Over 151,000 bottles of Apotex’s glaucoma treatment Brimonidine Tartrate/Timolol Maleate Ophthalmic Solution were recalled due to improper bottle sealing and potential lack of sterility of the 5mL and 10mL eye drops bottles.
On the same announcement, Apotex is recalling 493,000 bottles of 5mL prescription-only allergy relief eye drops Ketorolac Tromethamine because of improper bottle sealing and potential lack of sterility. Ketorolac Tromethamine drops are generic for Allergan’s Acular brand name version.
Both of these recalls are classified as a level III recall, the lowest urgency type of recall. The FDA has determined that product use is not likely to cause adverse health consequences. Check the FDA announcement for specific lot numbers.
FDA Recall Announcement

Dextroamphetamine Saccharate (Adderall XR Generic) Recall by Granules Pharmaceuticals
Over 50,000 bottles of Attention-deficit/hyperactivity disorder (ADHD) treatment Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate and Amphetamine Sulfate extended-release capsules have been recalled by Granules Pharmaceuticals because of an unknown impurity found in the product during a stability study.
This recall includes five separate lots of 100-count bottles with strengths ranging from 5 mg to 30 mg. Check the FDA announcement (Enforcement report) for the specific lot numbers. Adderall is used to treat ADHD. This recall is classified as a level II recall, the middle urgency type of recall because the probability of serious adverse health consequences is remote. This drug is generic for ADHD treatment Adderall XR.
FDA Recall Announcement

Lanett recently recalled its generic Adderall tablet version of the medication.
Read or add patient reviews for Adderall
Safety Update Planned for Acetaminophen
In a move that some medical groups do not agree with, the U.S. Food and Drug Administration has started the process for adding a warning to the labels of acetaminophen (Tylenol and similar products) to reflect evidence suggesting that the use of acetaminophen by pregnant women may be associated with an increased risk of neurological conditions such as autism and ADHD in children. The agency also issued a related letter alerting physicians nationwide.
In the letter, FDA Commissioner Martin Makary stated that “while an association between acetaminophen and autism has been described in many studies, a causal relationship has not been established and there are contrary studies in the scientific literature. The association is an ongoing area of scientific debate and clinicians should be aware of the issue in their clinical decision-making, especially given that most short-term fevers in pregnant women and young children do not require medication.”
Makary also reminds clinicians that acetaminophen is the safest over-the-counter medication option in pregnancy among all analgesics and fever reducers and that aspirin and ibuprofen have well-documented adverse impacts on the fetus.
The FDA provides links to large-scale studies, including the Nurses’ Health Study II and the Boston Birth Cohort that have results that suggest concerns. Some studies have described that the risk may be most pronounced when acetaminophen is taken chronically throughout pregnancy.
FDA to Study Potential Treatment for CFD-Related Autism
In line with its new commitment to finding the root causes of autism and treatments for it, the FDA is working with GSK, innovator of Wellcovorin (leucovorin calcium), to broaden the approval of leucovorin calcium tablets for patients with cerebral folate deficiency (CFD), a neurological condition that affects folate (a vitamin essential for brain health) transport into the brain.
Individuals with cerebral folate deficiency may have developmental delays with autistic features (e.g., challenges with social communication, sensory processing, and repetitive behaviors), seizures, and problems with movement and coordination. The FDA states that additional studies are needed to assess safety and efficacy before leucovorin calcium approval for this patient population.
Confusion about Folate Supplements and Leucovorin Calcium
Currently, leucovorin calcium is available as a generic only and is approved to relieve toxic effects of the cancer and rheumatoid arthritis (RA) drug methotrexate. Since the FDA’s announcement about its potential treatment for CFD, many people have been seeking over-the-counter versions of folate (Vitamin B9 or leucovorin) to help with autism symptoms. However, over-the-counter leucovorin is NOT the same drug as leucovorin calcium tablet, which is available by prescription only.
Washington Post: “Touted Treatment for Autism Sparks Interest in Over-the-Counter Alternatives”
Methotrexate patient reviews at Ask a Patient
Leucovorin calcium reviews
EMA Adds Warning about Eye Condition Causing Sudden Blindness to Ozempic and Wegovy Drug Labels
A rare but serious eye condition called non-arteritic anterior ischemic optic neuropathy (NAION) happens when blood flow to the optic nerve is blocked, causing sudden, painless loss of vision in one eye. It is a leading cause of vision loss in adults and the second most common optic neuropathy after glaucoma. The vision loss is generally irreversible, and there is currently no effective treatment available. In the past few years, there have been reports linking the condition to GLP-1 drugs like semaglutide and tirzepatide.
After a reviewing the evidence from non-clinical studies, clinical trials, and data from post-marketing surveillance, The European Medicines Agency (EMA) committee concluded that NAION is associated with use of semaglutide (the active ingredient in Ozempic and Wegovy). A required warning about the risk, which it classifies as very rare, affecting about 1 in 10,000 users, will appear on product labels for Ozempic and Wegovy.
In the European Union, patients are advised to immediately consult a doctor if they experience worsening eye sight or sudden loss of vision while using semaglutide and to discontinue use of semaglutide if they are diagnosed with NAION.
European Medicines Agency Press Release: “PRAC concludes eye condition NAION is a very rare side effect of semaglutide medicines Ozempic, Rybelsus and Wegovy”
The World Health Organization (WHO) also made an announcement about the risk of NAION associated with semaglutide. However, the EMA and WHO have not expanded the warning to include other GLP-1 diabetes and weight loss drugs such as tirzepatide-containing Mounjaro and Zepbound.
WHO Press Release: “The use of semaglutide medicines and risk of non-arteritic anterior ischemic optic neuropathy (NAION)”
The U.S. FDA has not yet announced any changes or potential changes to semaglutide labels related to NAION risk. According to the American Academy of Ophthalmology, U.S. ophthalmologists are NOT recommending discontinuation of GLP-1s for all patients who experience NAION, because of potential risk to some patients’ health. Instead, they are recommending a personalized decision making process.
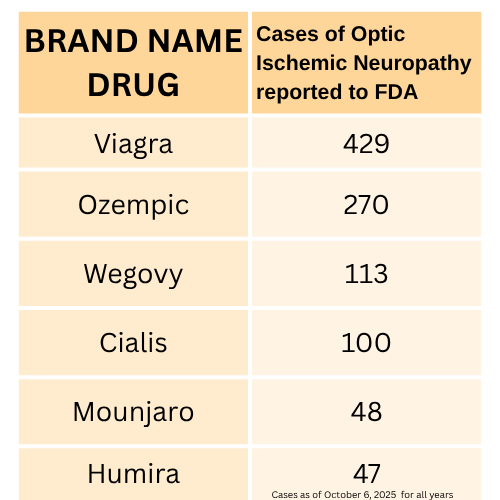
In 2024 and 2025, an increasing number of cases of ischemic optic neuropathy have been reported through the FDA adverse events reporting system (FAERS) for semaglutide drugs. Although patients with ischemic optic neuropathy (a broader term than NAION) may not experience the sudden blindness effects, this seems to be the closest “term” that is used to describe the NAION side effect in the FDA database.
In 2025 so far, the FDA has received 379 reports of ischemic optic neuropathy associated with Ozempic, Wegovy, and semaglutide combined. This represents 1.55% of all 24,484 semaglutide cases filed so far this year. In 2024, 106 cases were reported, and only a few reports with this side effect were submitted prior to 2024. The only drug with more reports of ischemic optic neuropathy is erectile dysfunction drug Viagra, with 429 total cases for all time.
Data Source: FDA FIS Dashboard
Read more about ischemic optic neuropathy and NAION at Cleveland Clinic.
Recommended Podcasts and Videos
In a recent episode of Sensible Medicine’s “This Fortnight in Medicine,” doctors Adam Cifu and John Mandrola discuss and interpret the findings from a study that examined how GLP-1 receptors in the optic nerves are affected by the GLP-1 receptor agonist drugs semaglutide and tirzepatide. The study found a link, albeit rare, between optic nerve eye disease (including NAION) and semaglutide and tirzepatide.
“Do diabetes medications help arthritis or hurt the eyes?” Listen or watch the episode here:
In the same program, the doctors give their opinion about a study suggesting that diabetes medicine metformin can help with osteoarthritis of the knee. Adam Cifu explains that he frequently prescribes metformin; in this episode he does a good job of describing, in layman’s terms, what metformin is.
First approved in Europe in the 1950s, metformin is emerging as a wonder drug (though not at the current level of GLP-1 excitement). Besides its use as a type 2 diabetes treatment, metformin has shown promise for treating Long Covid, improving longevity, lowering dementia risk for those with type 2, and as a treatment for kidney disease, lung conditions like COPD, and more.
UPI: “Metformin: Science finds new benefits from an old drug”
Interview with FDA Commissioner on Crackdown on Misleading Drug Ads and False Claims on Telehealth Websites
In a recent episode of investigative news program Full Measure, reporter Lisa Fletcher interviewed FDA Commissioner Marty Makary about new crackdowns on misleading drug ads and problematic telehealth websites like Hims and Hers. Also, Georgetown University’s Pharmed Out director Dr. Adriane Fugh-Berman explains how visuals in ads and cherry-picked safety disclosures are among the deceptive tactics that have been used for years to promote drugs directly to consumers. The segment is about five minutes long and aired on television September 28. Watch it here:
Full Measure: “Pharma Ads”
Note: It is now (on October 6) 4 business days beyond the 15-day deadline for pharmaceutical companies to respond to the warning letters sent by the FDA regarding misleading and distracting drug advertising. So far, most of the infringing television ads are still on the air, with no modifications or removals yet to address the FDA’s concerns. It could be that because of the government shut-down, pharma companies will gain some extra days to respond to the warnings.
Did you miss the last edition? Check it out here: