Skin Reactions and NSAIDs
Safety Labeling Changes for Prescription NSAIDs Warn of Rare but Severe Skin Reactions
It has long been recognized that drugs treating pain, fever and inflammation, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), can cause skin-related allergic reactions. The gastrointestinal system and skin are the most likely body systems to have an adverse reaction to NSAIDs. Skin reactions might include itch, hives, facial swelling, and sun sensitivity.
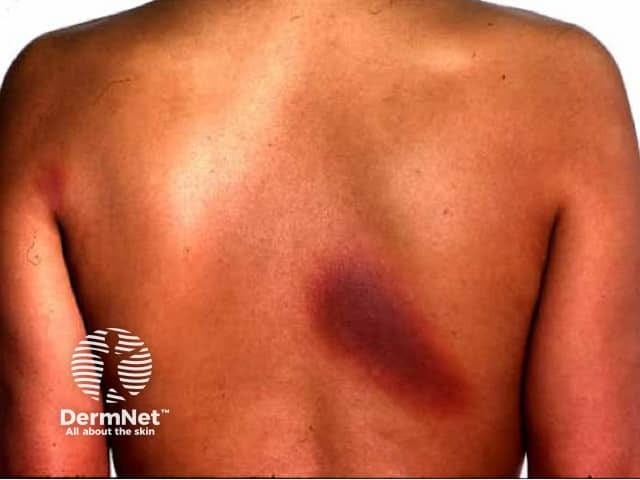
An unusual type of skin hypersensitivity reaction to an NSAID is "fixed drug eruption," which is a rash or flare that recurs in the same spot each time the person takes the same medication. (See image at end of this post.) It usually resolves on its own but may change the skin pigment even after the inflammation has subsided. This side effect is more common in adults than in children, according to DermNet.
A very serious form of fixed drug eruption is "generalized bullous fixed drug eruption," a life-threatening reaction that usually requires hospitalization. Based on the number of reports with this type of reaction coming into the FDA Adverse Events Reporting System, the FDA has decided to require that NSAID prescription drug labels include a warning about the risk of fixed drug eruptions, including generalized bullous fixed drug eruptions.
For each drug below, the new update warns of the risk of fixed drug eruption (FDE) and states that FDE may present as a more severe variant known as generalized bullous fixed drug eruption (GBFDE), which can be life-threatening. Please check the FDA’s Safety-Related Label Change Database (SRLC database) to search for additional NSAID drugs with the new warning.
Arthrotec (diclofenac sodium; misoprostol)
Arthrotec treats osteoarthritis and rheumatoid arthritis in adult patients at high risk of developing NSAID-induced gastric and duodenal ulcers. Arthrotec was initially approved in 1997.
Arthrotec FDA Safety Label Change and Revised Drug Label
Cambia (diclofenac potassium) powder
Cambia comes in one-dose packet of powder that is mixed with water. It treats attacks of migraine headaches in adults. Cambi was initially approved in 1998.
Cambia FDA Safety Label Change and Revised drug label
Celebrex (celexocib)
Celebrex treats osteoarthritis (OA), rheumatoid arthritis (RA), juvenile rheumatoid arthritis (JRA) ankylosing spondylitis, acute pain, menstrual cramps. It was initially approved in 1998.
Celebrex FDA Safety Label Change and Revised drug label
Daypro (oxaprozin) caplets
Daypro treats osteoarthritis (OA) and rheumatoid arthritis (RA) and was initially approved in 1992.
Daypro FDA Safety Label Change and Revised drug label
Duexis (famotidine; ibuprofen)
Duexis treats osteoarthritis (OA) and rheumatoid arthritis (RA) while decreasing the risk of developing an ulcer from ibuprofen use. Duexis was initially approved in 2011.
Duexis FDA Safety Label Change and Revised drug label
Feldene (piroxicam)
Feldene treats osteoarthritis (OA) and rheumatoid arthritis (RA) and was initially approved in1982.
Feldene FDA Safety Label Change and Revised drug label
Indocin (indomethacin) oral suspension and indomethacin (indomethacin) (various formulations)
Indomethacin treats moderate to severe rheumatoid arthritis (RA) including acute flares; ankylosing spondylitis; osteoarthritis; acute painful shoulder; gouty arthritis. Indomethacin was initially approved in1985 (oral suspension formulation) and in 1965 (capsules).
Indocin Safety Label Change and Revised Drug Label
Indomethacin FDA Safety Label Change and Revised drug label
Meloxicam (meloxicam) oral suspension
Meloxicam treats osteoarthritis (OA), rheumatoid arthritis (RA) and juvenile rheumatoid arthritis (JRA). It was initially approved in 2004.
Meloxicam FDA Safety Label Change and Revised Label
Naprosyn (naproxen) tablets and EC-NAPROSYN (naproxen) delayed release tablets, and ANAPROX DS (naproxen sodium) tablets
Naprosyn treats moderate to severe rheumatoid arthritis (RA); ankylosing spondylitis; osteoarthritis; bursitis; gout; menstrual cramps; pain management. Naprosyn was initially approved in 1976.
Naprosyn FDA Safety Label Change and Revised Drug Label (covers all 3 versions of naproxen)
Zipsor (diclofenac potassium) tablets
Zipsor treats mild to moderate pain in adults and children age 12 and up. Zipsor was initially approved in 1988.
Zipsor FDA Safety Label Change and Revised Drug Label
More Safety Information on NSAIDs:
Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | FDA
The FDA has released three drug safety communications about NSAIDs:
FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy
Example image of “fixed drug reaction” at Medline Plus.
Example image of “fixed drug bullous eruption,” which is a more severe form of fixed drug reaction at Medline Plus.
DermNet® article on fixed drug eruption: https://dermnetnz.org/topics/fixed-drug-eruption
Have you ever taken any of the meds mentioned in this article? Are you curious about how well they worked for others? Please read or add a review at Ask a Patient for any of the medications below:
ZIPSOR
Thank you for sharing your experience with other patients!
Note: Today’s post will be sent in two parts; the next section will include two drug recalls, an FDA safety communication, and ten new safety label updates.
