September 22, 2024: new drugs for MS, eczema; first-ever treatment for rare and devastating Niemann Pick childhood disease; non-drug capsule for weight loss; Neuralink nod; med tips and more
New Drug Approvals
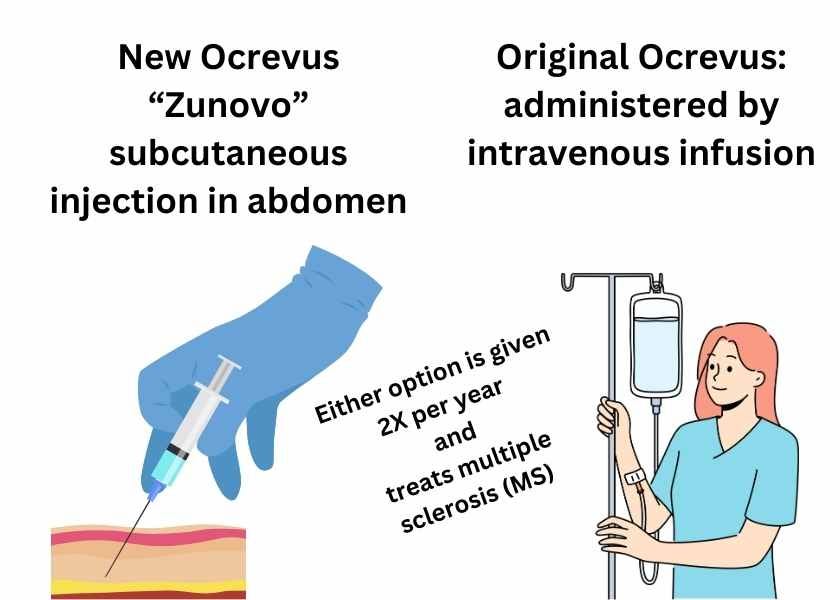
Ocrevus Zunovo for Multiple Sclerosis
Genentech’s multiple sclerosis drug Ocrevus Zunovo (ocrelizumab;hyaluronidase-ocsq) was approved by the Food & Drug Administration (FDA). Like regular Ocrevus (ocrelizumab), it treats relapsing forms of multiple sclerosis (MS) and primary progressive multiple sclerosis (PPMS). However, the Zunovo version is administered by subcutaneous injection in the abdomen twice a year instead of by intravenous infusion (IV) twice a year. Unlike regular Ocrevus, there is no initial 2-dose dosing regimen, making the treatment easier to start. The most common adverse reaction observed in clinical trials (experienced by 49% of patients) was injection reactions.
https://www.pharmacytimes.com/view/fda-approves-ocrevus-zunovo-for-2-forms-of-multiple-sclerosis
Ebglyss (lebrikizumab-lbkz)
Eli Lilly’s Ebglyss (lebrikizumab-lbkz) subcutaneous injection was approved to treat moderate to severe eczema in patients age 12 and up (weighing at least 88 lbs or 40kg). The most common Ebglyss side effects are injection site pain or reactions, shingles (herpes zoster), and eye and eyelid inflammation and itching. The prefilled pen doses are injected monthly as maintenance doses after an initial more frequent dosing regimen. The medication may be used with or without topical therapies.
Ebglyss Press Release
Miplyffa (arimoclomol)
Zevra Therapeutics’ Miplyffa (arimoclomol) was approved as the first medication for the treatment of neurological symptoms associated with Neimann-Pick disease, type C (NPC) in combination with the enzyme inhibitor miglustat in patients age 2 and older. NPC is a rare genetic disease that results in progressive neurological symptoms and organ damage; those afflicted rarely live more than 13 years. Capsule contents are mixed with water, apple juice, apple sauce, yogurt or pudding. Arimoclomol had previously been studied as a treatment for ALS but was not successful in meeting its efficacy goals. Miplyffa’s application was submitted and then withdrawn from the European Union’s European Medical Agency.
FDA Press Release for Miplyffa
Read more about the different types of NPC at Medline Plus:
https://medlineplus.gov/genetics/condition/niemann-pick-disease/
Kisqali Use Expanded to include Early Stage Breast Cancer
Novartis’ Kisqali (ribociclib) and Kisqali Femara Co-Pack received expanded approval for the treatment of adults with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative stage II and III early breast cancer at high risk of recurrence.
https://finance.yahoo.com/news/fda-approves-novartis-kisqali-reduce-165500419.html
Autoimmune Drugs Tremfya, Cimzia, Dupixent
Tremfya (guselkumab) received an expanded approval to treat ulcerative colitis (US). Cimzia and Dupixent also had expanded approvals for pediatric use. See today’s Drug Safety Updates newsletter from today for more information.
FluMist Nasal Spray Influenza Vaccine Approved for Self-Administration
MedImmune’s FluMist influenza vaccine live intranasal spray was approved as the first vaccine for self-administration (or caregiver-administration) at home. FluMist is approved for use by individuals 2 through 49 years of age. For the self-administration option, the vaccine manufacturer plans to make it available through a third-party online pharmacy. Medimmune is part of AstraZeneca.
FluMist Press Release
While you can’t yet order it for at-home use, FluMist is available this season (2024-2025) from some pharmacies and healthcare providers. Check out Ask a Patient’s new flu information page for a description of what’s new this flu season. For instance, did you know that all vaccines this year are trivalent (covering 3 viruses) instead of quadrivalent (covering 4 infuenza virus strains)?
https://www.askapatient.com/news/flu-vaccines-for-2024-2025-season.asp
Medical Devices: Neuralink’s Blindsight
Neuralink was granted the FDA’s "breakthrough device" designation for its experimental implant that aims to restore vision. This allows it to move into human trials. Neuralink founder Elon Musk says that the device, called Blindsight "will enable even those who have lost both eyes and their optic nerve to see.” Blindsight works by implanting a chip directly into the visual cortex of the brain.
Neuralink is not the only team working on the technology. A small number of blind people around the world have already risked brain surgery to get a visual prosthesis. In Spain, researchers at Miguel Hernández University have implanted four people with a similar system. The visual cortex’s location at the back of the head makes it easily accessible for an implant by performing a routine craniotomy to remove a piece of the skull. Check out Wired’s interview with a completely blind 56 year-old patient who volunteered to receive the experimental visual cortex implant.
https://www.wired.com/story/the-next-frontier-for-brain-implants-is-artificial-vision-neuralink-elon-musk/
Medical Device: “Epitomee” Drug-free Capsule for Weight Loss
The FDA cleared Epitomee Medical’s capsule, to be taken in combination with lifestyle modification, for weight management in adults with a BMI starting at 25. Once swallowed, the capsule expands in the stomach, creating a sensation of fullness. After several hours, the device moves to the intestines where it disintegrates and passes from the body through the digestive tract.
https://www.empr.com/news/fda-clears-epitomee-an-ingestible-device-for-weight-management/
This animated video from Epitomee Medical shows how the device works:
Video Link to Epitomee
In case you missed our special report earlier this month, Cephalon (Teva) quietly discontinued the pain drug category called TIRF (Transmucosal Immediate Release Fentanyl). No new patients may receive the meds, and current patients may only continue to receive them until supplies run out. The meds include immediate release cancer pain medications Actiq (the “lollipop” pain med), Fentora lozenge, and fentanyl citrate. Read our report and view product images:
https://www.askapatient.com/news/tirf-pain-meds-fentanyl-discontinued.asp
Statins and Older Patients: Needed or Not?
Does it make sense for older patients to take lipid lowering medications like statins? Anthony C. Pearson, MD, FACC., also known as “The Skeptical Cardiologist,” says that two large ongoing trials are trying to answer the question. In the meantime, he recommends that:
“If you are >75 years of age and free of any clinical atherosclerotic cardiovascular disease (ASCVD) there is little evidence that taking a statin drug will benefit you.”
Dr. Pearson also suggests getting a coronary artery calcium scan, because “very low or zero scores lower your individual risk for ASCVD and substantially higher than average scores increase your risk.” Read all of his recommendations, along with his humorous musings about alternatives for the term “elderly” in the medical literature.
https://theskepticalcardiologist.com/2024/09/07/are-you-too-old-to-worry-about-high-cholesterol/
High Doses of ADHD Amphetamines and Psychosis Incidence
Could higher doses of ADHD drugs (prescription amphetamines) increase the risk of psychosis? An analysis of 1,374 emergency room visits of patients age 16 - 35 that involved a psychosis diagnosis found that patients who had taken high doses of Adderall (more than 40 mg) or 100 mg of Vyvanse (lisdexamfetamine) had a five-fold increase in cases than the control group. 40 mg is the maximum recommended dose for Adderall use for ADHD, according to its drug label. The dosage for treating narcolepsy is between 5 and 60 mg per day. For Vyvanse, the maximum recommended dosage is 70 mg once daily.
Joe Graedon of the People’s Pharmacy describes important take-aways from the study, which was published in the American Journal of Psychiatry.
https://www.peoplespharmacy.com/articles/unmasking-risks-hidden-dangers-of-adhd-mental-health-meds
Podcast Recommendation: “Less Radical” (the story of cancer research pioneer Bernie Fisher)
Not that long ago, the standard of treatment for any diagnosis of breast cancer was a complete mastectomy (removal of both breasts). As host Dr. Stacy Wentworth explains in her new podcast, the work of pioneering surgeon-scientist Bernie Fisher led to far less radical treatments. Tune in on Wednesday, September 25 for the launch of the first of six podcasts that tell the story of Dr. Bernie Fisher, who not only revolutionized breast cancer treatment, but also fundamentally changed the way we understand all cancers. “Less Radical” will be presented in six episodes and is available on all podcast outlets. Listen using the link below:
After working well into his 90s, Dr. Fisher died five years ago in October 2019 at the age of 101. In this essay, oncologist Stacy Wentworth explains why Bernie Fisher deserves a biography and what led her to write one.
https://cancerletter.com/cancer-history-project/20240809_3/
Medication and Supplement Tips
Dr. Lucy McBride answers a reader question about whether you really have to take Vitamin K and D together for the Vitamin D to work. She also answers a question from a 60-year-old woman who has had success with HRT (hormone replacement therapy) and wonders if she needs to stop taking it. Dr. McBride also answers a third question: “What is the optimal blood pressure for diabetics?” Check out the Q&A:
If you prefer, you may Read the transcript.
Natural Treatments Tip: Apple Cider Vinegar
Can apple cider vinegar really improve your health? Lindsey Wohlford, a registered dietitian at the University of Texas MD Anderson Cancer Center, shares her opinions with the American Heart Association regarding four health claims about apple cider vinegar.
https://www.heart.org/en/news/2024/09/11/what-can-apple-cider-vinegar-really-do-for-your-health
Visit us at AskaPatient.com for ratings and reviews provided by patients along with more news and health information.
Did you miss this week’s Drug Safety Updates? It covers nine drugs. Check it out:
https://news.askapatient.com/p/september-2024-part-2-drug-safety-veozah-more



