March 18, 2024: New drug for common liver disease; 4 cancer drugs, OTC continuous glucose monitor, Prolia generic, Puberty blockers blocked; 2023 generics report; how drug samples may affect care
New Drug Approvals
The U.S. Food and Drug Administration (FDA) approved Madrigal Pharmaceutical's Rezdiffra (resmetirom) for patients noncirrhotic non-alcoholic steatohepatitis (NASH) with moderate to advanced liver scarring (fibrosis). NASH, a common liver disease, is a result of the progression of nonalcoholic fatty liver disease where liver inflammation, over time, can lead to liver scarring and liver dysfunction. It is often associated with other health problems such as high blood pressure and type 2 diabetes. Along with diet and exercise, Rezdiffra can reduce scarring and inflammation in people with fatty accumulations in their livers. “Previously, patients with NASH who also have notable liver scarring did not have a medication that could directly address their liver damage,” said Nikolay Nikolov, M.D., acting director of the Office of Immunology and Inflammation in the FDA’s Center for Drug Evaluation and Research.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease
The FDA approved Novo Nordisk's Wegovy (semaglutide) as a treatment to reduce the risk of cardiovascular death, heart attack and stroke in adults with cardiovascular disease and either obesity or overweight. Wegovy should be used in addition to a reduced calorie diet and increased physical activity. Wegovy is also approved to reduce excess weight and maintain weight reduction long term in certain adults with obesity or overweight and certain children with obesity, for use in addition to a reduced calorie diet and increased physical activity.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-reduce-risk-serious-heart-problems-specifically-adults-obesity-or
The FDA approved BeiGene's Tevimbra for treating esophageal squamous cell carcinoma (ESCC) after previous systemic chemotherapy. The FDA is reviewing another Tevimbra application in first-line esophageal cancer, with a decision expected in July.
https://www.fiercepharma.com/pharma/beigene-finally-snags-fda-approval-pd-1-drug-tevimbra-after-long-delay-novartis-breakup
Cancer Drugs: Expanded Approvals
The FDA approved Bristol-Myers Squibb Company's nivolumab (Opdivo,) in combination with cisplatinc and gemcitabine for first-line treatment of adult patients with metastatic urothelial carcinoma (UC).
The FDA approved Pfizer's inotuzumab ozogamicin (Besponsa) for pediatric patients 1 year and older with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia.
The FDA expanded the approval of BeiGene’s Brukinsa (zanubrutinib) as a treatment for advanced cases of follicular lymphoma. It’s the fifth FDA approval for the drug, which has become BeiGene’s top-selling product. BeiGene’s twice-daily capsule is administered alongside periodic infusions of obinutuzumab, brand name Gazyva, a targeted therapy from Roche that is a standard follicular lymphoma treatment. https://medcitynews.com/2024/03/beigene-cancer-drug-fda-approval-brukinsa/
Pediatric Approvals
The FDA expanded the use of cholesterol drug Praluent (alirocumab) as an adjunct to diet and other low density lipoprotein cholesterol (LDL-C)-lowering therapies in pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH) to reduce LDL-C. Clinical trials information was added to the drug label.
https://www.empr.com/home/news/praluent-approved-for-pediatric-patients-with-heterozygous-familial-hypercholesterolemia/
Praluent ratings and reviews at Ask a Patient website
Puberty Blockers Blocked in U.K.
The U.K.'s National Health Service (NHS) announced that puberty blockers will no longer be prescribed to minors under age 18 in England outside of regulated clinical trials. The NHS called for an independent review of gender identity treatments for minors in 2020 amid a “significant increase” in referrals to the Gender Identity Development Service, which is scheduled to close at the end of March.
https://www.forbes.com/sites/tylerroush/2024/03/12/uk-bans-puberty-blockers-for-minors/?sh=3ec55b312a3b
In 2022, Sweden ended the practice of prescribing puberty blockers and cross-sex hormones to gender-dysphoric patients under the age of 18. Last month, Ask a Patient Health News: Drugs & Treatments reported on changing sentiments in Europe that have resulted in a more cautious approach to prescribing the drugs.
Continuous Glucose Monitoring Device Approval (OTC)
The FDA approved Dexcom’s Stelo Glucose Biosensor System, the first continuous glucose monitor (CGM) to be sold over-the-counter. It is intended for use by those 18 years and older who do not use insulin, such as individuals with diabetes treating their condition with oral medications, or those without diabetes who want to better understand how diet and exercise may impact blood sugar levels. The system is not to be used by individuals with problematic hypoglycemia (low blood sugar). The device utilizes a wearable sensor and smartphone app to continuously monitor glucose levels, displaying readings every 15 minutes. Each sensor lasts up to 15 days before needing replacement.
https://www.fda.gov/news-events/press-announcements/fda-clears-first-over-counter-continuous-glucose-monitor
Generic Drug News
The FDA released its 2023 annual report from the Office of Generic Drugs. A total of 956 generics were approved in 2023, with 90 of those being “first generics,” where no generic competition previously existed. Generics for R.A. drug Xeljanz, ADHD drug Vyvanse, and breast cancer drug Ibrance were among the notable first generics of 2023. Download the full 26 page report.
Patient ratings of Xeljanz, Patient ratings of Vyvanse,
Patient ratings of Ibrance and safety information

The FDA approved Sandoz’ Jubbonti (denosumab-bbdz) injection as the first interchangeable biosimilar to osteoporosis drug Prolia (denosumab), and Wyost (denosumab-bbdz) injection as the first interchangeable biosimilar to Xgeva (denosumab) for preventing skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors. Both Jubbonti and Wyost are administered by subcutaneous injection and may be taken along with calcium and vitamin D as necessary to treat or prevent low calcium levels.
https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-first-interchangeable-biosimilars-prolia-and-xgeva-treat-certain-types-osteoporosis-and
Recently, independent testing lab Valisure was awarded a contract with the U.S. Defense Department (DoD) to do medication quality testing. Along with multiple study partners, they are launching a Pharmaceutical Quality Risk Assessment Study to independently study the quality of generic drugs in the United States and create a “Red/Yellow/Green” rating system to help industry purchasers and patients avoid products with concerning results. Consumers are encouraged to submit their suggestions for particular generic drugs to be included in the study:
https://www.valisure.com/valisure-newsroom/vote-participate-in-the-largest-independent-pharmaceutical-quality-study
Did you know that the amount of active ingredient in a drug, whether brand or generic, can vary – by as much as 10% less or up to 10% more than the amount indicated on the label? This is a surprise to many people. Read more at AskaPatient about ingredients in generic drugs and what it takes to be considered “bioequivalent.”
https://www.askapatient.com/news/generics-differing-from-brands-ingredients-explainer.as
Research News: Device for Generating Speech from Throat Movements
Bioengineers at the University of California, Los Angeles, have built a soft, adhesive patch, adhered the skin over the throat, capable of turning muscle movements of the larynx into audible speech. The self-powered technology could serve as a non-invasive tool for people who have lost the ability to speak due to vocal cord problems. After collecting data on laryngeal muscle movement, researchers used a machine-learning algorithm to correlate the resulting signals to certain words.
The research team demonstrated the system’s accuracy by having participants (eight volunteers) pronounce five sentences — both aloud and voicelessly — including “Hi, Rachel, how are you doing today?” and “I love you!” The overall prediction accuracy of the model was 94.68%, with the participants’ voice signal amplified by the actuation component, demonstrating that the sensing mechanism recognized their laryngeal movement signal and matched the corresponding sentence the participants wished to say. In the future, they plan to continue enlarging the vocabulary of the device through machine learning and to test it in people with speech disorders. The study was published in Nature Communications.
https://newsroom.ucla.edu/releases/speaking-without-vocal-cords-ucla-engineering-wearable-tech
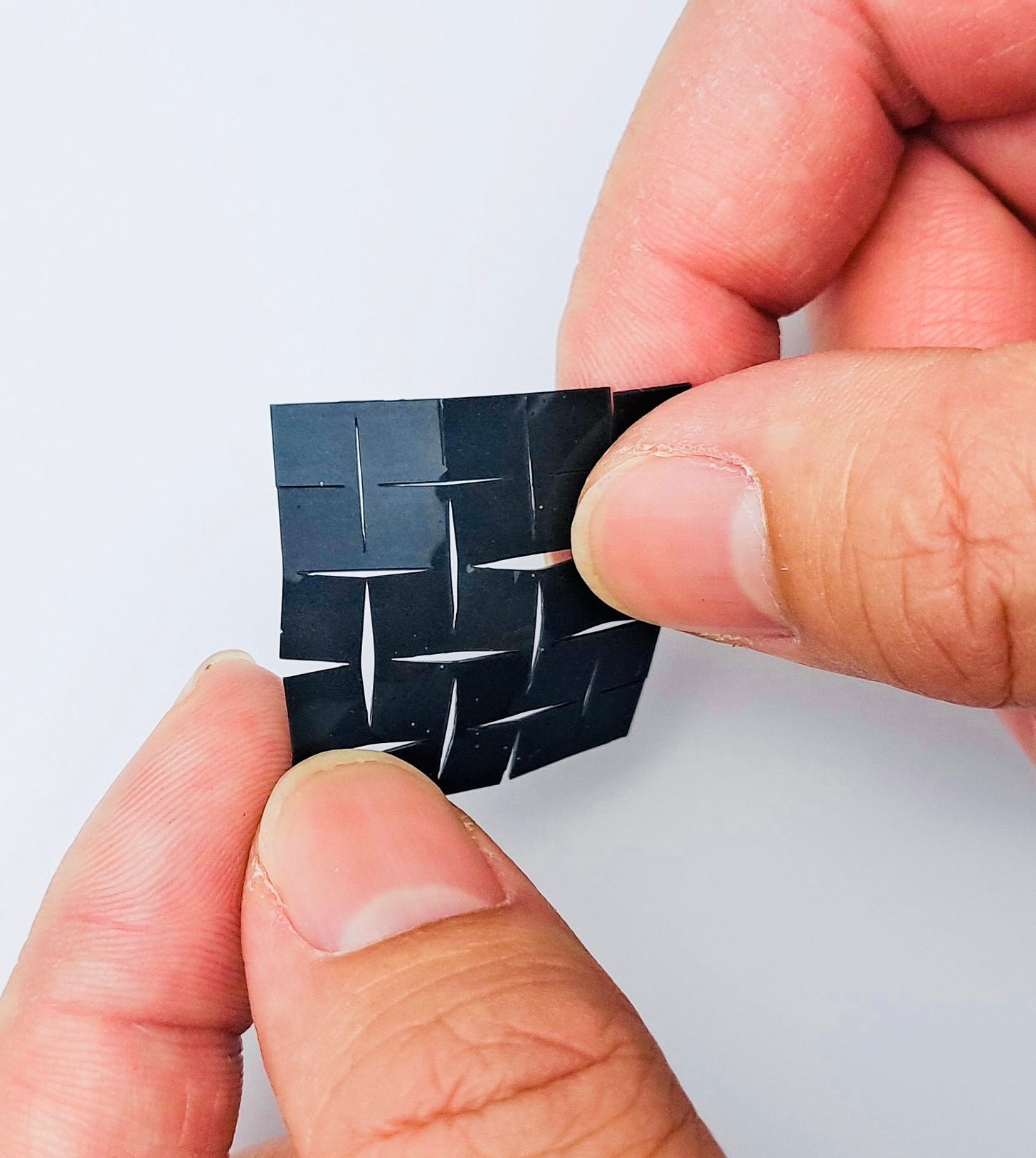
The breakthrough is the latest in lead researcher Jun Chen’s efforts to help those with disabilities. His team previously developed a wearable glove capable of translating American Sign Language into English speech in real time to help users of ASL communicate with those who don’t know how to sign.
Podcast Recommendation: Pharmaceutical Samples
Drug samples given to doctors by drug reps are not considered "gifts," the way meals and trips are, but they are a powerful marketing mechanism for pharmaceutical companies. The latest “Pharmanipulation” podcast features an interview with Shahram Ahari MD, an emergency medicine physician and former drug rep, exploring the world of pharmaceutical samples. PharmedOut’s Dr. Fugh-Berman and Caroline Renko chat with Dr. Ahari about why samples are the most important marketing tactic drug companies have, how samples are used to manipulate prescribing choices, and discuss whether or not drug samples should be banned. This program is 31 minutes long.
Nutritional Supplement Tip: Fish Oil
Learn about research backing up the health benefits of fish oil, along with tips on dosage amounts, how often to take it, whether to take it with food, and advice about possible medication interactions and allergic reactions.
https://www.singlecare.com/blog/best-time-to-take-fish-oil/


