February 2025 Drug Safety Updates Part 1: Enhertu, Omvoh, Ozempic expanded approvals and new warnings; MS drug's new boxed warning; glaucoma eye drops and corneal swelling; Xtandi choking risk; more
Copaxone (glatiramer acetate) subcutaneous injection
Multiple sclerosis (MS) treatment Copaxone added a new boxed warning about the rare hypersensitivity risk of anaphylaxis. The new warning states that anaphylaxis can occur at any time, from as early as right after the first dose or up to years after starting the medication. Symptoms of anaphylaxis include wheezing or difficulty breathing, swelling of the face, lips, or throat, and hives. These symptoms can quickly progress to more serious symptoms, including severe rash or shock, which is a life-threatening condition. The new safety warning also applies to the generic glatiramer acetate and the branded generic Glatopa. Copaxone was approved in 1996.
Copaxone FDA Safety Label Change
FDA Announcement: FDA Press Release and Drug Safety Communication on Boxed Warning
Ratings and Reviews for Copaxone
For an explanation of the difference between regular (unbranded), authorized, and branded generics, read this article on AskaPatient: "Generic Drug Types.”
Depakote (divalproex sodium) and Depakote Extended Release capsules
Depakote is an anticonvulsant that treats seizure disorders; it is also sometimes prescribed for bipolar disorder or to prevent migraines. Anticonvulsants work by reducing the excessive firing of neurons that happens in a seizure. Depakote's label was updated to include the risk of drug-induced hyperpigmentation, or dark patches on the skin. Depakote was initially approved in 1989.
Depakote FDA Safety Label Change
Ratings and Reviews for Depakote and Depakote ER
Enhertu (trastuzumab deruxtecan) intravenous infusion
Breast cancer drug Enhertu had a label update to support its expanded approval for treating HER2-low or ultralow (earlier stages) of breast cancer, as determined by an FDA-approved antibody test. The "Pathway HER2" antibody test was specifically designed to screen patients who are likely to respond to Enhertu.
The label includes new clinical trials results indicating that there were no differences in in efficacy in patients age 65 and older treated for HER2-low or ultralow breast cancer compared to younger patients treated for the same types of cancer. However, there was a higher incidence of Grade 3-4 adverse reactions observed in those older patients being treated for HER2-low or ultralow compared to younger patients. Grade 3 is considered severe and a Grade 4 adverse reaction is life threatening according to the NLM grading scale of adverse events. Enhertu was approved in 2019.
Enhertu FDA Safety Label Change
FDA Press Release on Enhertu Expanded Approval
Leqembi (lecanemab-irmb) intravenous infusion
Leqembi is a recently approved monoclonal antibody treatment for patients with early stage of Alzheimer's disease. It is used to reduce amyloid beta plaque, a protein found in the brain of people with Alzheimer's disease. The boxed warning was modified to add that the severe side effect of ARIA (Amyloid Related Imaging Abnormalities) can be fatal. ARIA-E (for edema/effusion) often includes temporary swelling in areas of the brain. It usually occurs early in treatment and is asymptomatic, although serious and life-threatening events like seizures can occur.
New guidance for clinicians advises that because ARIA-E can cause symptoms that can mimic an ischemic stroke, practitioners should consider whether such symptoms could be due to ARIA-E before giving thrombolytic (stroke) therapy to a patient being treated with LEQEMBI. Leqembi was approved in 2023.
Leqembi FDA Safety Label Change
Read more about Leqembi and Alzheimer’s at MedLine Plus.
Norditropin (somatropin recombinant) subcutaneous injection
Norditropin is a human growth hormone used to treat children with growth hormone deficiency or short stature associated with various conditions including Noonan or Turner syndrome. Noridtropin also treats adults with growth hormone deficiency. The label was updated to add the adverse event of gynecomastia (breast enlargement) and to clarify medication refrigeration storage instructions. Norditropin was initially approved in 1987.
Norditropin FDA Safety Label Change
Ratings and Reviews for Norditropin
Omvoh (mirikizumab-mrkz) injection (intravenous or subcutaneous)
Ulcerative colitis drug Omvoh updated its label to support its expanded approval to treat Crohn’s disease. Clinical trials results were added along with a list of common side effects experienced by people using the medication for Crohn's disease. These include upper respiratory infections, elevated liver blood tests, joint pain, injection site reactions and headache. Omvoh was approved in 2024.
Omvoh FDA Safety Label Change
Ozempic (semaglutide) subcutaneous injection
Type 2 diabetes drug Ozempic had a label revision to support its expanded approval as a treatment to reduce the risk of worsening of kidney disease and to reduce the risk of cardiovascular death in adults with type 2 diabetes and chronic kidney disease. The label added warnings about post-approval reports of acute pancreatitis (including fatal and non-fatal cases), inflamed gallbladder, dysesthesia (abnormal sense of touch), alopecia (hair loss), and severe gastrointestinal adverse reactions. The Patient Medication Guide adds that symptoms of acute pancreatitis include severe abdominal pain that may radiate to the back, which may or may not be accompanied by vomiting.
Ozempic FDA Safety Label Change
Ratings and Reviews for Ozempic
Qelbree (viloxazine) extended-release capsules
Qelbree is a non-stimulant medication that treats Attention Deficit Hyperactivity Disorder (ADHD) in adults and pediatric patients age six and up. It is a selective norepinephrine reuptake inhibitor (SNRI), a type of drug also used to treat depression (Cymbalta and Effexor are SNRIs).
Qelbree's label was updated to provide the results of a lactation study showing that the drug passes into breast milk at a low level. At this time, there are no data on viloxazine effects on the breastfed infant or the effects on milk production. Qelbree was approved in 2021.
Qelbree FDA Safety Label Change
Ratings and Reviews for Qelbree
Rocklatan (latanoprost; netarsudil dimesylate) ophthalmic drops
Rocklatan treats open-angle glaucoma or ocular hypertension. Its label was updated to warn that one of its ingredients, netarsudil, has been associated with epithelial corneal edema (corneal swelling), described as honeycomb or bullous. Patients are advised to notify their physician if they experience eye pain or decreased vision while using Rocklatan. Epithelial corneal edema typically resolves upon discontinuation of the medication. Rocklatan was initially approved in 2019.
Rocklatan FDA Safety Label Change
Case study reporting the adverse effect of epithelial corneal edema with use of netarsudil drops.
Versacloz (clozapine) oral suspension and Clozaril (clozapine) oral suspension
Versacloz and Clozaril are used for treatment-resistant schizophrenia. The labels were updated to add the risk of pericarditis to boxed warnings about potentially fatal cases of myocarditis and cardiomyopathy. Pericarditis is a condition in which the sac-like covering around the heart (pericardium) becomes inflamed, while myocarditis is an inflammation of the heart muscle itself. Myocarditis and pericarditis most frequently occur within the first two months of clozapine treatment. The list of symptoms of myocarditis and pericarditis was updated to include “heart palpitations.” Also, fecal incontinence was added to the post-approval list of reported side effects. Clozapine was approved in 1989.
Versacloz FDA Safety Label Change and Clozaril FDA Safety Label Change
Ratings and Reviews for Versacloz and Clozapine and Clozaril
Read about pericarditis at Medline Plus.
Xtandi (enzalutamide) capsules and tablets
Prostate cancer drug Xtandi added a new section to its label warning of the risk of dysphagia (difficulty swallowing) or choking because of the size of the product. Patients are advised NOT to cut, crush, or chew the product and to take each capsule or tablet whole with a sufficient amount of water to ensure that all medication is successfully swallowed. Patients should inform their healthcare provider if they are unable to swallow the prescribed product.
According to the product description section, the 80mg oblong tablets are 17 mm in length, while the 40mg round tablets are just 10 mm wide. The 40 mg oblong capsules are about the length of a penny at 20mm long. Xtandi was approved in 2012.
Xtandi FDA Safety Label Change
Ratings and Reviews for Xtandi
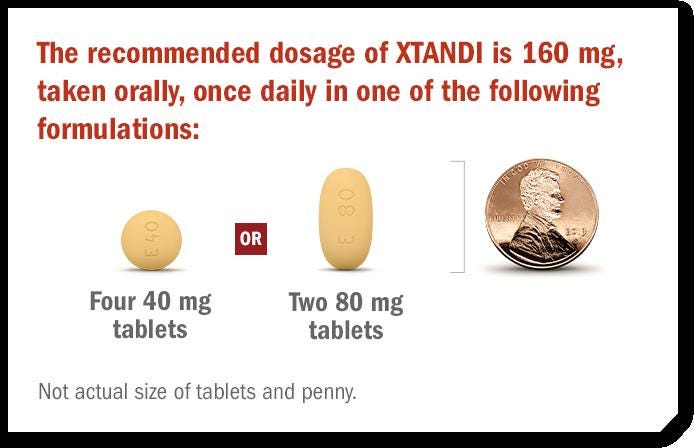
It would not be surprising if many patients prefer the option of taking four of the small pills instead of just two of the penny-sized versions of enzalutamide. A study on patient preferences (“Patients' evaluation of shape, size and colour of solid dosage forms”) concluded that gelatin capsules are preferred over tablets for ease of swallowing. The ideal tablet is coated, small and white, with arched and circular shape. The study (from 2001) was published in Pharmacy World and Science.
Vertex's Journavx (suzetrigine) was recently approved for treating moderate to severe acute pain, such as pain resulting from injury or surgery. Pain News Network's Pat Anson explains why the evidence suggests that suzetrigine does not compare with opioid pain relievers and is a mild pain reliever, at best.
Pain News Network: "FDA Approves New Non-Opioid Pain Reliever"
FDA Press Release : “First Drug Approved in New Class of Non-Opioid Pain Medicines”
Thank you for reading this edition of Drug Safety Updates. Check out our website at www.askapatient.com for patient ratings and reviews of prescription drugs. Coming soon: our 2024 drug approvals highlights.
Did you miss our last two newsletters? Check them out here:
Note to readers: Due to the large number of safety label updates recently posted by the FDA, you will receive a bonus edition of Drug Safety News next weekend.



