Drug Safety Updates: March 2024
Notable FDA Safety announcements and label changes
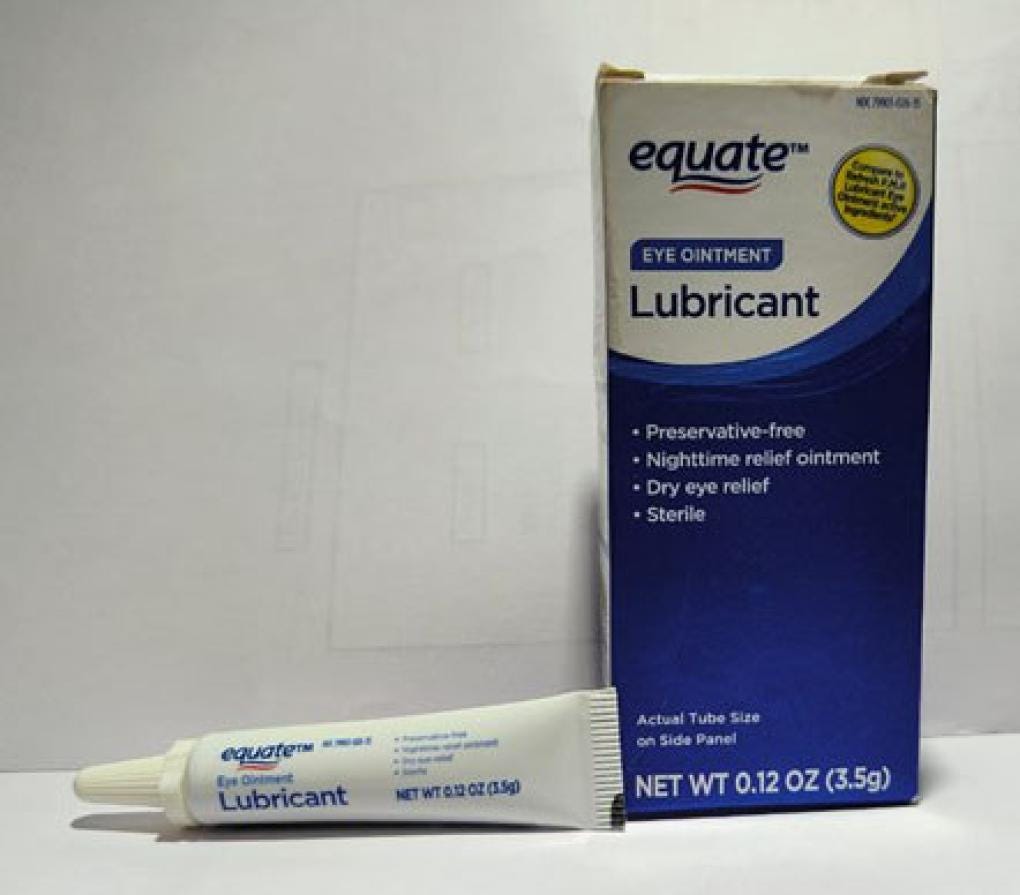
Eye Ointment Safety Recall
Brassica Pharma Pvt. Ltd. issued a nationwide recall of certain eye ointment products due to an FDA inspection which found unsanitary conditions at the manufacturing facility in India. The affected products include Equate Lubricant Eye Ointment and Equate Stye Lubricant Eye Ointment, as well as CVS Health Lubricant Eye Ointment and Lubricant PM Ointment. Expiration dates range from February 2024 to September 2025. Consumers are advised to stop using the recalled products immediately and return them to the place of purchase. As of February 16, Brassica Pharma Pvt. Ltd. has not received any reports of adverse events related to this recall.
Check this link for full product list and product photos.
https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/brassica-pharma-pvt-ltd-issues-voluntary-nationwide-recall-equate-lubricant-eye-ointment-equate-stye
Drug Safety Label Updates
Beyfortus (nirsevimab-alip), the recently approved monoclonal antibody drug given to infants to prevent against severe cases of RSV, had its drug label updated to report additional severe hypersensitivity reactions.
https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=2920
Covid-19 drug Veklury (remdesivir) had its drug label updated to report the results of clinical trials for pediatric subjects. Its drug label was also updated to reflect that it is now also approved for children under the age of 12 weighing at least 1.5 kg. who are either hospitalized or not hospitalized and are at high risk for progression to severe COVID-19.
https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=2411
Multiple Myeloma Drug Withdrawn
The FDA announced its final decision to withdraw approval of Pepaxto (melphalan flufenamide), which was granted an "accelerated approval" in February 2021 for use in combination with dexamethasone to treat certain patients with multiple myeloma, a type of blood cancer. The agency determined the following grounds for withdrawal were met: (1) the confirmatory study conducted as a condition of accelerated approval did not confirm Pepaxto’s clinical benefit, and (2) the available evidence demonstrates that Pepaxto is not shown to be safe or effective under its conditions of use.
According to its most recent drug label, which contains post-approval clinical trials results, 6% of patients receiving Pepaxto died from adverse events, with general physical health deterioration and respiratory failure being the most common causes, and serious adverse reactions occurred in 49% of patients who received Pepaxto.
https://www.fda.gov/drugs/drug-safety-and-availability/fda-issues-final-decision-withdraw-approval-pepaxto-melphalan-flufenamide
Dietary Supplements Safety
The FDA renamed its dietary supplements "Ingredient Directory" as "Information on Select Dietary Supplement Ingredients and Other Substances." The updated directory now includes 80 ingredients that have been involved in various types of FDA safety-related actions or communications. These include ingredients subject to authorized or qualified health claims, ingredients that have been the topic of safety communications, and ingredients that meet or do not meet various standards and definitions in the federal Food, Drug, and Cosmetic Act (FD&C Act).
Examples include Acadibol, calcium, cannabidiol, Diclofenac, DHA, red yeast rice, Vardenafil, and Psyllium husk.
https://www.fda.gov/food/dietary-supplements/information-select-dietary-supplement-ingredients-and-other-substances
