Drug Safety Labeling Updates: July 2024: Metronidazole and risk of severe skin reactions and tinnitus; Xeomin cosmetic treatment; more
Another batch of antibiotics with new warnings about allergic reactions
Metronidazole antibiotics: severe skin reactions and tinnitus
This month, the U.S. Food and Drug Administration (FDA) published another round of antibiotics safety label updates, this time for the antibiotic metronidazole (Flagyl brand name). (In case you missed it, read last month’s report about sulfa-type antibiotics’ safety label changes.) Metronidazole is an anti-protozoal antibiotic, and treats a variety of infections including amebiasis, sexually transmitted infections like trichomoniasis, bacterial vaginosis (BV), dental abscess, appendicitis, stomach ulcers, plus a variety of other bacterial infections. The label has a boxed warning stating that metronidazole should only be used as directed for approved indications because it has been shown to cause cancer in laboratory animals (mice and rats).
Two changes were made to metronidazole drug labels:
Skin-related severe adverse reactions
Strengthened warnings about hypersensitivity risk of severe allergic skin reactions, or "severe cutaneous adverse reactions" (SCARs):
"Severe cutaneous adverse reactions (SCARs) including toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of metronidazole. Symptoms can be serious and potentially life threatening."
Patients are advised to discontinue the medication immediately and take appropriate therapy if signs or symptoms of SCARs develop.Hearing-related side effects
Warnings were added about the risk of tinnitus, hearing impairment, and hearing loss.
This side effect had not appeared on the label previously, but patients have been reporting hearing-related side effects associated with metronidazole on Ask a Patient website for years.
This batch of label updates for metronidazole-containing drugs includes capsules, tablets and solutions (not topical creams or gels). Click the link to go to the drug label update on the FDA website. (some of the duplicate generics have been removed from the list below; the label updates are the same.)
FLAGYLFLAGYL I.V. RTU IN PLASTIC CONTAINER
METRO I.V. IN PLASTIC CONTAINER
METRONIDAZOLE IN PLASTIC CONTAINER
PYLERA
COMMON SIDE EFFECTS:
In addition to nausea and headache, one of the most commonly reported side effects for metronidazole is metallic taste or funny taste in the mouth.
Read some of these taste-related problems experienced by patients taking
metronidazole or Flagyl.
The U.S. FDA reported drug safety label updates for 14 antibiotic medications containing metronidazole. (Image source from: Dailymed, NIH)
Cosmetic drug label warns about use with certain pre-existing facial conditions; bruising at injection site
Xeomin (incobotulinumtoxinA), an injectable botulinum toxin product similar to Botox is used to help temporarily approve the appearance of facial lines in adults, had a label update instructing to "Use caution when XEOMIN is used where the targeted muscle shows excessive weakness or atrophy." This includes:
”patients who have marked facial asymmetry, with surgical alterations to the facial anatomy, pre-existing eyelid or eyebrow ptosis, when excessive weakness or atrophy is present in the target muscles, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin (e.g., the inability to substantially lessen glabellar lines even by physically spreading them apart).”
In addition, the side effect of "injection site bruising" was added to the drug label.
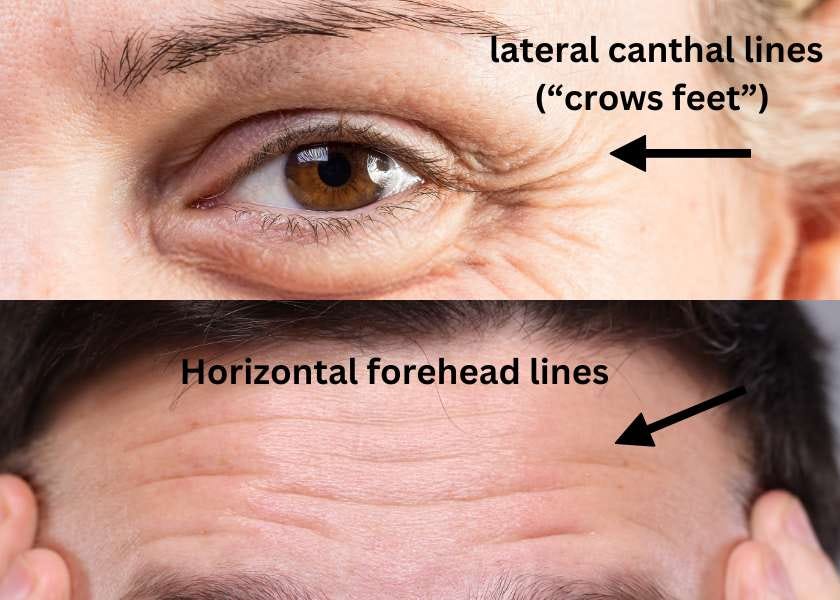
Xeomin is also now approved for the additional indication of upper facial lines (which include horizontal forehead lines, and lateral canthal lines)
FDA drug safety labeling supplement for Xeomin.
Three drugs warn of risk of intrahepatic cholestasis of pregnancy
Two leukemia drugs, Purinethol (mercaptopurine) and Thioguanine (thioguanine) had drug safety label updates to warn of postmarketing reports of intrahepatic cholestasis of pregnancy (ICP), a liver disorder that occurs in the second or third trimester. With ICP, a build-up of bile in the liver impairs its function, causing intense itching and sometimes causing serious pregnancy complications. These cases occured when the drugs were used for inflammatory bowel disease (IBS). The labels state that the drugs are NOT indicated to treat inflammatory bowel disease.
PURINETHOL drug safety label update
THIOGUANINE drug safety label update
Immunosuppresant drug Imuran (azathioprine), also had an update to its label to warn of the risk of intrahepatic cholestasis of pregnancy. Imuran is used to prevent kidney transplant rejection and is also used to treat severe rheumatoid arthritis.
The label says that “ICP symptoms and evaluated bile acid levels improved following azathioprine discontinuation.” Furthermore, the label says that Imuran should be avoided during pregnancy, as it may harm the fetus.
Read the entire IMURAN drug safety label update
Read more about the liver disorder intrahepatic cholestasis of pregnancy (ICP) on MedlinePlus. The article includes an illustration of the intrahepatic bile duct anatomy.
https://medlineplus.gov/genetics/condition/intrahepatic-cholestasis-of-pregnancy/
Learn about the general liver disorder cholestasis on MedlinePlus:
https://medlineplus.gov/ency/article/000215.htm
Thanks for reading this drug safety update! Looking for our regular newsletter? Our “Drugs & Treatments” edition will be sent as a “Quick Takes” (brief link descriptions) tomorrow, July 15.