December 10, 2023: First CRISPR gene editing therapy approved; cancer risk with some gene therapy; epilepsy drug warning; CVS to change how it prices drugs; osteoporosis and flu drugs reviews
Also, big study on long-term use of ADHD stimulants and cardiac issues; lung cancer and depression podcast recommendations
New Drug Approvals
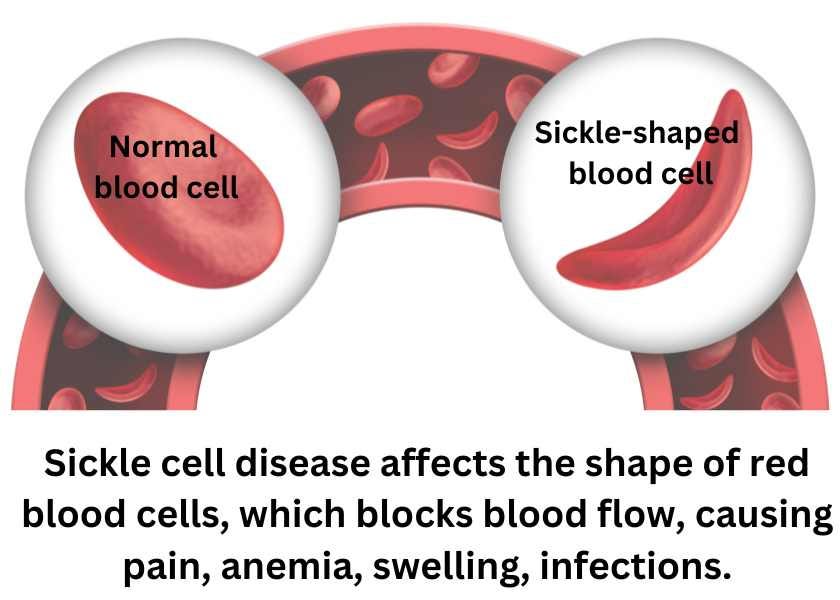
On Friday, the U.S. Food & Drug Administration (FDA) approved two treatments for sickle cell disease. Vertex Pharmaceuticals' Casgevy is the first-ever CRISPR/Cas9 gene editing treatment to receive FDA approval. Also approved was Bluebird Bio's Lyfgenia, a cell-based gene therapy. Both treatments are multi-step, multi-million dollar procedures that require a hospital stay. The steps involve collecting the patients’ own stem cells, making the genetic alterations to the stem cells, high-dose chemotherapy to prepare bone marrow so that it can receive the cells, and infusion with the modified cells. Casgevy was approved in the United Kingdom in November and is expected to be approved by the European Medical Association (EMA) and elsewhere.
https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease
Lyfgenia has a black box warning about the risk of blood cancer. Biospace reports that Vertex's Casgevy will have a U.S. list price of $2.2 million per patient, while Bluebird set an even steeper list price for Lyfgenia in the U.S. at $3.1 million per patient.
https://www.biospace.com/article/casgevy-vs-lyfgenia-the-battle-of-the-sickle-cell-gene-therapies-has-begun/
Check out our article explaining sickle cell disease and Vertex's CRISPR/Cas 9 technology:
https://askapatientnews.substack.com/p/gene-editing-explained-crispr-treatment
The FDA expanded the approval of Eli Lilly's Jaypirca (pirtobrutinib) blood cancer drug.
FDA Press Release
The FDA approved Novartis’s Fabhal capsules to treat a rare blood disorder paroxysmal nocturnal hemoglobinuria. The drug has a boxed warning about the risk of severe infections from encapsulated bacterial infections like Streptococcus pneumonia.
https://medcitynews.com/2023/12/fda-approval-novartis-rare-blood-disorder-fabhalta/
Drug Safety News
Could cell-based gene therapies meant to cure cancer also cause cancer?
The FDA is investigating whether CAR-T immunotherapy, a type of cell-based gene therapy, causes secondary cancers, including lymphoma. CAR-T therapies include Abecma (idecabtagene vicleucel), Breyanzi (lisocabtagene maraleucel) Carvykti (ciltacabtagene autoleucel) Kymriah (tisagenlecleucel) Tecartus (brexucabtagene autoleucel) and Yescarta (axicabtagene ciloleucel). These treatments, all approved within the last six years, treat various blood cancers.
https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-investigating-serious-risk-t-cell-malignancy-following-bcma-directed-or-cd19-directed-autologous
As Wired reports, "Scientists use harmless viruses to ferry and insert the new genetic material because of their natural ability to get inside cells. But the potential for these viruses to accidentally trigger another cancer has long been considered a theoretical risk."
Fierce Pharma has a chart by CAR-T drug name with counts of cancer cases, serious cancer cases, and deaths reported in the FDA Adverse Event Reporting System (FAERS) database.
https://www.fiercepharma.com/pharma/fda-investigates-serious-risk-secondary-cancer-following-car-t-therapy-treatment
The FDA issued a safety warning about a rare condition linked to epilepsy/seizure medications levetiracetam (brand names Keppra and Spritam) and clobazam (Onfi, Sympazan) Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), may start as a rash with swollen lymph nodes in the face but can quickly progress, resulting in injury to internal organs, the need for hospitalization, and even death. Symptoms of DRESS generally start 2 weeks to 8 weeks after starting on the medicine, but these symptoms may occur earlier or later. Medication guides and labels will be updated with this information.
https://www.fda.gov/safety/medical-product-safety-information/antiseizure-medicines-keppra-keppra-xr-elepsia-xr-spritam-levetiracetam-and-onfi-sympazan-clobazam
These drugs had safety label updates:
Prostate cancer drug Xtandi's label was updated to include side effects like muscle and joint pain, tiredness, bleeding problems, falls, headache; clinical trials results were also updated into the label. FDA label changes for Xtandi.
Prostate cancer drug Trelstar’s label was updated to warn that the use of GnRH agonists may lead to metabolic changes such as hyperglycemia, diabetes mellitus, and hyperlipidemia. Non-alcoholic fatty liver disease, including cirrhosis, occurred in the post-marketing setting. FDA label changes for Trelstar.
Weight loss drug Contrave has a new adverse warning for Brugada pattern/syndrome (a heart syndrome). FDA label changes for Contrave.
Osteoporosis drug Bonsity label was updated to warn of incidence of osteosarcoma (a malignant bone tumor). FDA label change for Bonsity.
Thyroid drug Tirosint had a safety label update regarding pediatric use and adverse consequences of overtreatment or undertreatment. FDA label change for Tirosint.
CVS to Change its Prescription Drug Price Formula
CVS announced it is rolling out a new drug cost pricing model called CostVantage starting in 2024. Its pricing formula is based on the cost of the drug, a set markup and a fee for filling the prescription. Some say this sounds a lot like Mark Cuban's Cost Plus Drugs business model while others think the CVS plan is different since it apparently will negotiate a mark-up % with each health plan. Industry analyst Adam Fein predicts that other large pharmacies will likely follow CVS with some form of cost-plus reimbursement model.
Inc. reports that Mark Cuban's Cost Plus drugs really is starting to "disrupt" the drug industry. It now sells over 1,000 generics and 10 brand-name medications through a network of independent pharmacies in 38 states and through its flagship online pharmacy. Earlier this year, Blue Shield of California switched its Pharmacy Benefit Manager (PBM) from CVS Caremark to Cuban's Cost Plus.
https://www.inc.com/jeff-haden/cvss-latest-move-proves-mark-cuban-is-truly-disrupting-drug-industry.html
Public Citizen Health Research Group Drug Reviews
Public Citizen's Health Research Group (HRG), a consumer watchdog organization, reviewed the injectable osteoporosis drug romosozumab (Evenity). Although Evenity has been FDA-approved since 2019 for postmenopausal women who are at high risk of fractures and for whom other osteoporosis therapies have not worked, HRG classified it as a "Do Not Use" drug. Their medical review team says that its limited benefits do not outweigh the serious cardiovascular risks, including heart attack and stroke, associated with the drug. Additionally, HRG does not recommend the continuous use of bisphosphonates (like Boniva; Evenity is not a bisphosphonate) for more than five years to minimize the risk of potential adverse events, which include pain, fractures, and osteocrenosis of the jaw.
https://www.worstpills.org/newsletters/view/1570 (this article is paywalled; abstract provided).
Patient reviews of Evenity at Ask a Patient website
Based on a "meta-analysis" of scientific studies, researchers at McGill University concluded that oseltamivir (Tamiflu) was no better than placebo at reducing hospitalizations or preventing complications (such as serious bacterial infections) that may occur during or after influenza infections. The study was published in JAMA Internal Medicine. HRG notes that the benefits of oseltamivir, which has been on the U.S. market for almost 25 years, are limited to a small reduction in flu symptoms. Public Citizen classifies Tamiflu (and generic oseltamivir) as a "Limited Use" drug.
https://www.worstpills.org/newsletters/view/1568 (this article is paywalled; abstract provided)
On Ask a Patient website, patients report a wide range of experiences with Tamiflu, with many experiencing worsening nausea while others reporting a fast recovery if they taken within a very short time after getting symptoms.
Long-term use of ADHD Stimulants and Hypertension
A Karolinska Institute-led study of 278,027 individuals in Sweden aged 6 to 64 years taking ADHD medication found that over the long-term, patients are at a higher risk for cardiovascular disease (CVD), particularly hypertension and arterial disease. The study, which utilized the Swedish National Inpatient Register and the Swedish Prescribed Drug Register, found the incidence of cardiovascular disease to be higher for patients using stimulant rather than nonstimulant ADHD medications. Stimulant medications included methylphenidate (example Concerta), amphetamine (Adderall), dexamphetamine (Dexedrine), and lisdexamfetamine and nonstimulants were atomoxetine (Strattera) and guanfacine (Intuniv). Across the 14-year follow-up, each 1-year increase of ADHD medication use was associated with a 4% increased risk of CVD.
https://www.medicalnewstoday.com/articles/certain-adhd-medications-may-increase-heart-disease-risk
Cough Drops Advice
Did you know that cough drops containing menthol can actually make your cough WORSE, especially when taken many times a day and for too many days? Check out MedShadow Foundation's "The Cough After the Cold."
https://medshadow.org/cough-drops/
Podcast Recommendations
Brain Stimulation for Severe Depression
Dr. Joshua Gordan of National Institute of Mental Health (NIMH) interviewed Dr. Sarah Hollingsworth “Holly” Lisanby, an internationally recognized innovator in Electroconvulsive therapy (ECT), about brain stimulation tools as treatments for severe depression. Lisanby describes how "seizure therapies" were performed in the 1940s and how techniques have changed since then. She also explains the technology of transcranial magnetic stimulation (TMS). Check out "Mental Health Matters Podcast: The Evolution of Brain Stimulation Therapy." The podcast is 16 minutes long and a transcript is available.
https://www.nimh.nih.gov/news/media/2023/the-evolution-of-brain-stimulation-therapy
Read more about TMS with links to resources on AskaPatient website: https://www.askapatient.com/news/transcranial-magnetic-stimulation-rtms-for-depression.asp
Lung Cancer Treatments
Aging Matters host Cheryl Beversdorf interviewed Eric Anderson, MD, Director, Interventional Pulmonology, MedStar Georgetown University Hospital and Associate Professor Georgetown University, about lung cancer prevalence, risk factors, diagnosis, treatment and prevention. He explains why most cases of lung cancer are discovered in people over the age of 65 and at later, less-treatable stages. Many asymptomatic people meet the criteria for yearly screening. A painless low radiation dose CT scan might reveal some very small tumors that won't involve any drug treatments. He discusses big advances in lung cancer treatments, including targeted therapies for certain mutations and immunotherapy. This podcast is 54 minutes long.
A podcast by cardiologist John Mandrola may be of interest if you have atrial fibrillation (Afib) because he discusses three big changes in American AF treatment guidelines.
Visit us as AskaPatient.com for drug ratings and reviews provided by patients along with more news and health information.
Note to readers: Ask a Patient® Health News (general edition) will be sent on Wednesday, December 13. If you are not already subscribed to this separate newsletter, sign up today on our web site.