Caplyta (lumateperone) Capsules: How a New Approval Can Change a Drug Label in Unexpected Ways
In the Drug Safety Updates newsletter, we usually report on an expanded FDA approval or revised drug safety information with a description and also a link to the associated drug label change. Today, we’re taking a closer look at a drug label change to show how risk or guidance information might be edited and even removed in a revision, sometimes without an explanation as to why the changes are being made. Drug companies write the labels for the products they sponsor; an exception is when the FDA requires that certain safety notices be added to a label. Mainly, the FDA reviews and approves the company-written drug labels.
After comparing Caplyta’s “old” (June 2023) drug label with the newly revised label (November 2025), it is notable that the Tardive Dyskinesia Warning Section was scaled back, even though movement-related side effects were more prevalent compared with placebo in the trials for the new indication. We won’t get into the clinical trial effectiveness results here (which were favorable), as we are only looking at the adverse events warning changes.
Background
Caplyta (lumateperone, 42mg) was first approved in 2019 as an “atypical antipsychotic” treatment for schizophrenia. It later received approval for bipolar disorder in adults and also as an add-on treatment for bipolar-related depression to be used either alone or along with lithium (a mood stabilizer) or valproate (an anticonvulsant). The drug was developed by Intracellular Therapies, but was purchased by Johnson & Johnson (J&J) in early 2025 for $14.6 billion.
New Indication for Caplyta: Major Depression
On November 5, 2025, Caplyta received expanded FDA approval as a treatment for major depressive disorder (MDD) in adults, to be used along with an antidepressant. Adults who don’t respond to an antidepressant alone (any type) may now be prescribed the antipsychotic as an adjunctive treatment. Fierce Pharma notes that this new indication will “super-size the market for Caplyta, as MDD affects about 22 million adults each year, according to J&J. . .which is anticipating Caplyta will eventually bring in $5 billion in annual sales.”
The clinical trial studies that supported its approval (Study 501 (NCT04985942 and Study 502 (NCT05061706) for major depressive disorder were six weeks long and compared Caplyta/Antidepressant with a Placebo/Antidepressant combination. The third study (called Study 503 with the code NCT0561719) was only open to patients who had actually completed one of the first two studies. That study was open label (all patients took Caplyta plus an antidepressant) and looked at long-term (six months) efficacy and safety. Data for that long-term trial is not yet included in the drug label.
Caplyta’s revised drug label, which includes a Patient Medication Guide, adds that the most common side effects reported in the MDD study were dizziness, drowsiness, and sedation. Importantly, the “Patient Medication Guide” warning about Tardive Dyskinesia was kept intact, and remained the same as the 2023 version.
Movement Disorder Risks
However, with no apparent connection to the new MDD indication or research, the main label content (section 5.5) on the risk of “Tardive Dyskinesia” (TD) was revised. Although second-generation antipsychotics like Caplyta generally have less movement-related side effects like tremors, muscle spasms, gait disturbances, and tardive dyskinesia than first-generation drugs, the risk still exists. Also, Caplyta is so new that long-term side effect risks are still unknown.
The “Tardive Dyskinesia” risk section is noticeably shorter (about half the length) compared with the previous version. It removes the definition of the condition (“Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs”), and moves the warning about the relationship between long-term use and the increased likelihood of it being an irreversible condition into a paragraph about increased risk of TD in elderly women. The new label also removes the paragraph cautioning that prescribing should happen in a manner that is “most likely to reduce the risk of tardive dyskinesia.” Together, these revisions seem to weaken the strength of the warnings about TD.
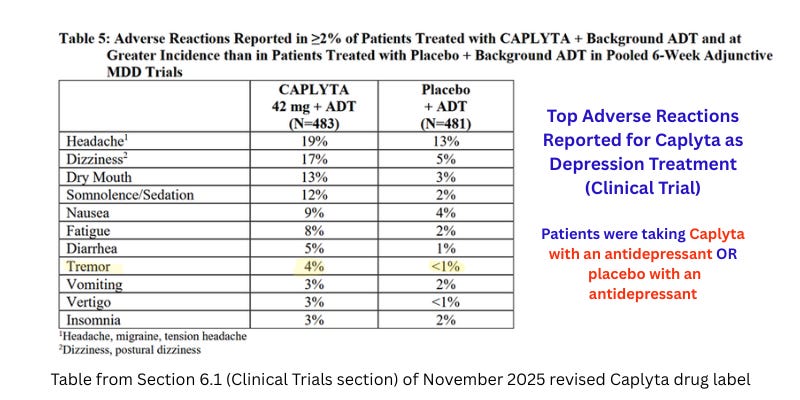
The revisions to the TD warning section are surprising, considering that the MDD clinical trial had a higher prevalence of extrapyramidal (movement-related) side effects for Caplyta + antidepressant vs. placebo + antidepressant than did the trials for other indications (bipolar-related depression and schizophrenia). For the MDD study, the clinical trial adverse events summary states:
“In the 6-week, adjunctive MDD trials, the frequency of reported EPS-related adverse reactions (tremor, bradykinesia, muscle spasms, gait disturbance, tongue spasm, muscle tightness and dyskinesia), excluding akathisia and restlessness, was 5% for CAPLYTA-treated patients and 0.8% for placebo-treated patients.”
Compare this with the EPS (movement related) types of adverse events occurring in the trials for the other approved indications:
For the schizophrenia trials, the frequency of EPS-related reactions was 6.7% for the CAPLYTA-treated patients and 6.3% for placebo-treated patients.
(Could it be that some of the schizophrenia patients participating in the Caplyta trial had already developed TD from previous years of medication, so that the difference in the prevalence of this adverse effect between the tested drug and placebo was not as great as in the MDD trial, which likely included patients who had never taken an antipsychotic before?)For the monotherapy bipolar depression trials, the frequency of reported EPS-related reactions was 1.3% for CAPLYTA-treated patients and 1.1% for placebo-treated patients.
(These results suggest that the antidepressant-Calypta combo may be more likely to result in the EPS-related reactions than the antidepressant or Caplyta alone (see below).For the adjunctive bipolar depression trials (where lithium or valproate was also used) the frequency of reported EPS-related reactions was 4% for CAPLYTA-treated patients and 2.3% for placebo-treated patients.
(Because the difference was less than 2%, that adverse reaction was not included in the clinical trial adverse event comparison table. Also, the result suggests that the antidepressant-Calypta combo may be more likely to result in the EPS-related reactions than the antidepressant or Caplyta alone.)
The below chart is from the most recent drug label, providing clinical trials adverse reactions for the MDD trials. Note the “Tremor” adverse reaction of four percent for patients taking Caplyta:
A Takeaway
While this drug has been “on a roll” and much celebrated in the media, safety concerns exist. The additional approval means that a huge new patient population, most likely without prior antipsychotic experience, will soon be taking this drug. Based on the MDD clinical trials results, it seems like a new “movement-related” warning should have been added to the Patient Medication Guide for the MDD indication.
Even if the longer terms trials show that the movement related disorders go away eventually or are not serious, it should be disclosed that the effect can happen early in treatment. Many patients don’t look at the clinical trial results and won’t know about this new risk unless their doctor has thoroughly read the drug label and shares the information with a patient considering taking the new medication.
In addition, while we don’t know whether the FDA questioned what appears to be a weakening of the warnings section on Tardive Dyskinesia, it would be helpful if the drug sponsor’s reasoning for all significant labeling changes were required to be included in the publicly accessible FDA letters to drug sponsors. For now at least, the television/video ads include the same warnings about TD that existed before the label update. However, the other types of movement disorders that occurred during the MDD trials are not mentioned in the commercials that promote the drug as an add-on for treating depression.
Sources and More Reading
Caplyta Drug Label Safety Update (November 2025) and links to current and previous drug labels.
Cleveland Clinic: “Antipsychotic Medications”
Psychiatric Times: Interview with Principal Investigator (Intra-Cellular Therapies) for Caplyta
Fierce Pharma “JJS Caplyta Supersizes Its Reach with Lucrative Expansion for Major Depressive Disorder”
FDA: “FDA Changes to an Approved Drug” and “Frequently Asked Questions about Labeling for Prescription Medications”
Clinical Trials are linked within this article.
Ratings for Caplyta: Patient Opinions About Caplyta (Please add yours!)