Are patients happy with Ozempic if they aren't aiming to lose weight?; new antidepressant; MS treatment; disappointing "supplemental" approvals; good & bad news for Covid drugs; food as medicine
Diabetes Drugs News
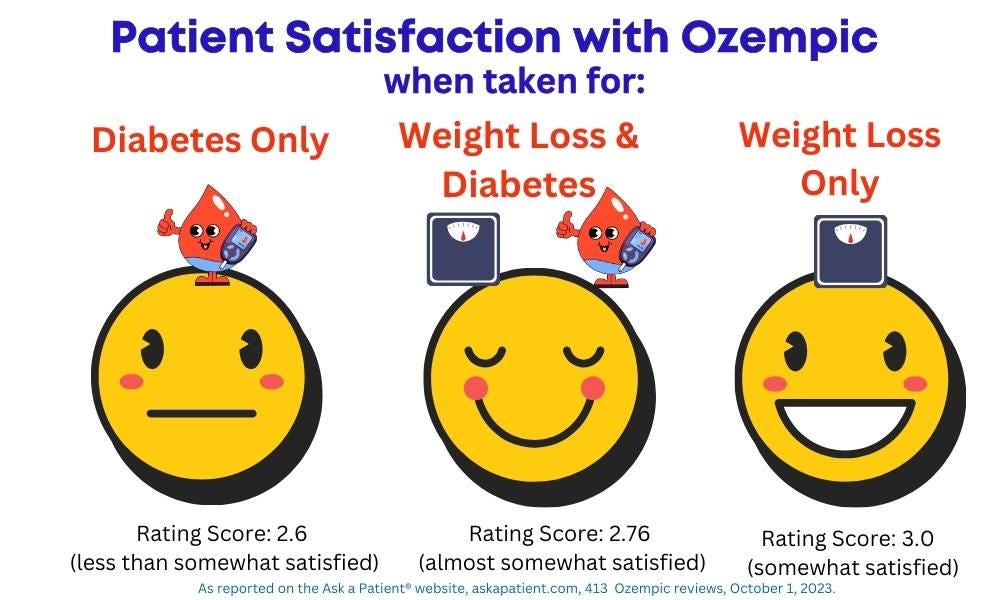
The recent buzz about Ozempic (semaglutide) has been on weight loss rather than on how well it works for its approved purpose – to lower diabetes' patients' A1C (blood sugar) levels. For patients who are not focused on weight loss and are instead aiming to lower their A1C, how does the drug stack up? We compared satisfaction ratings on the Ask a Patient® web site for those who reported taking Ozempic for diabetes-related reasons only (206 patients), for weight loss only (98), and those taking it for both reasons: to lose weight and for diabetes (101 patients).
The results showed that patients are happiest with the drug when they are aiming to lose weight, with about a half a point improvement in satisfaction score compared with when the goal is only to improve blood sugar levels. Satisfaction scores are on a scale of 1 to 5, with 1 being least satisfied, 3 is somewhat satisfied, and 5 is the highest rating level.
Read over 400 patient reviews of Ozempic on Ask a Patient.
Novo Nordisk's Ozempic had two safety label updates to warn of the risk of "ileus," or obstruction of the small intestine, a condition that occurs when the intestines are unable to contract to keep food moving through the digestive tract. The label was also updated to stress the risk of severe hypoglycemia (drop in blood sugar levels) when taken in conjunction with insulin products. Weight loss drug Wegovy (semaglutide), has not yet filed a similar update.
https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=2183
Read more about the safety issue of ileus & stomach paralysis with Ozempic and Wegovy at People’s Pharmacy:
https://www.peoplespharmacy.com/articles/how-scary-is-stomach-paralysis-with-ozempic-and-wegovy
Check out PharmedOut's latest podcast episode called “Fat and Fiction: Considering the risks and benefits of weight loss and weight loss drugs." Join hosts Dr. Adriane Fugh-Berman and Judy Butler as they interview activist Ragen Chastain and Dr. Joel Lexchin on weight-neutral healthcare, weight loss, and weight loss drugs including Wegovy and Ozempic. Pharmanipulation explores how industry markets not only drugs, but diseases, and exposes ineffective or harmful practices in medicine. This episode is 33 minutes long.
Diabetes drug Jardiance (empagliflozin) received a U.S. Food & Drug Administration (FDA) approval for a new indication: to reduce the risk of decline in end-stage kidney disease, cardiovascular death, and hospitalization in adults with chronic kidney disease. Jardiance also treats Type-2 diabetes and heart failure.
https://www.mmm-online.com/home/channel/fda-greenlights-jardiance-for-treating-chronic-kidney-disease/
Boehringer Ingelheim updated the label for diabetes drug Jardiance with a new section on the risk of lower limb amputation along with some other changes:
https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=323
Antidepressant Approval
The FDA approved Fabre-Kramer Pharmaceuticals' Exxua (gepirone) to treat major depressive disorder in adults. The approval of these extended-release tablets are a long time coming: it was rejected in 2004, 2007, and 2015 after an advisory panel voted against it due to lack of evidence of effectiveness. Fabre-Kramer is required to complete post-approval studies to provide a long-term safety assessment and pregnancy exposure data. Fabre-Kramer described Exxua as inaugurating a "new class of antidepressant” that will be available in pharmacies in 2024. Exxua is also being studied for the treatment of generalized anxiety disorder (GAD) and hypoactive sexual desire disorder. Gepirone is a relative of anxiety drug Buspirone (Buspar).
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021164
More on Exxua from Pharmacy Times:
https://www.pharmacytimes.com/view/fda-approves-gepirone-extended-release-for-treatment-of-major-depressive-disorder
Multiple Sclerosis Research
Uppsala University researchers found that stem cell transplants using a patient’s own stem cells to “reset” their immune system slowed the progression of relapsing–remitting multiple sclerosis (MS) and suggest that it be considered as a standard-of-care for severe disease. Autologous haematopoietic stem cell transplantation (aHSCT) works by using blood cells harvested from bone marrow to kick start immune systems after chemotherapy and is often used to treat blood cancers.
In the study, researchers analyzed data from a Swedish MS registry, investigating the safety and efficacy of aHSCT when used in routine healthcare settings – which are more representative of the general population – rather than clinical trials. Among the 149 patients who had some disability to begin with, 54% improved, 37% remained stable and 9% got worse. On average, patients were relapsing 1.7 times in the year before the aHSCT treatment. The study was published in The Journal of Neurology Neurosurgery & Psychiatry.
https://www.technologynetworks.com/biopharma/news/stem-cell-transplants-may-safely-slow-ms-progression-suggests-study-379121
Subsequent FDA Approvals for the Same Drug Tend to Have Less Therapeutic Value than the Original Approval
Once a drug is approved for the first time, it is common for the pharmaceutical company to seek additional (or supplemental) FDA approvals for the drug. For example, Pembrolizumab (Keytruda brand name) was originally approved in the US and Europe for the treatment of advanced melanoma. Later, it was approved for treatment of non-small cell lung cancer, head and neck squamous cell cancer, breast cancer, renal cell carcinoma, and other cancer indications. In the U.S., almost half of all drugs approved since 2011 have received at least one supplemental approval.
University of Zurich researchers analyzed the therapeutic value of supplement approvals made by the FDA and the European Medicines Agency (EMA) from 2011-2020 and found that fewer than half of these supplemental approvals add substantial value over existing treatments and only around a third of supplemental approvals add substantial therapeutic value compared with first approvals. Each subsequent supplemental approval was less likely to have a high therapeutic value rating.
The authors used the therapeutic value ratings for first and supplemental indications published by France's Ministry of Health and Germany's Federal Joint Committee. Not used in the study was data from England's National Institute for Health and Care Excellence, which only publishes the decision on coverage, not a rating, and the Canadian Human Drug Advisory Panel, which only provides a rating for the first indication but not subsequent approvals. No U.S. agencies were identified that publish therapeutic value ratings.
The authors conclude that when first or supplemental indications do not offer added therapeutic value over other available treatments, that information should be clearly communicated to patients and physicians and reflected in the price of the drugs. The study was published in the BMJ.
https://scienmag.com/fewer-than-half-of-new-drugs-add-substantial-therapeutic-value-over-existing-treatments/
Allergies to Penicillin are Vastly Over-Reported
Millions of people wrongly believe they are allergic to penicillin, which could mean they take longer to recover after an infection, according to the Royal Pharmaceutical Society (RPS). Tase Oputu, from the RPS, said: "Many individuals are at low or very low risk of having a genuine penicillin allergy and we often find that after careful investigation that they can take penicillin safely." A childhood allergy from years ago may actually have been a side effect or may no longer be an issue. She urged anyone in that position to ask questions about their allergy next time they visit their doctor. About four million people in the UK have the drug allergy on their medical record - but when tested, 90% of them are not allergic, research suggests.
https://www.bbc.com/news/health-66934909
Penicillin drugs include amoxicillin, Augmentin 875, and others. Check them out on AskaPatient.com.
Good and Bad News in Recent Studies of Covid Drugs
The People's Pharmacy's Joe Graedon reports on the good and bad news as revealed in two new studies about Covid antiviral medications Paxlovid and Lagevrio. The good news is that for vulnerable patients, based on a study by Cleveland Clinic and published in JAMA Network Open, the drugs still seem to reduce the likelihood of hospitalization. The bad news is that the benefits were somewhat lower than the original clinical trials suggested.
The other study, by researchers at Francis Crick Institute, Cambridge University, and others and published in Nature, is bad news for Lagevrio (molnupirivir). The research confirms that Covid virus can survive treatment with molnupirivir, then trigger mutations which sometimes show up in other patients. Molnupirivir maker Merck has criticized the research, saying it has not yet been proven the new mutations came from patients treated with Lagevrio.
https://www.peoplespharmacy.com/articles/covid-update-the-pros-and-cons-of-paxlovid-and-legevrio
Read 40 patient experiences with Paxlovid on Ask a Patient.
Food as Medicine
Check out MedShadow Foundation's new article on beets and beetroot supplements. Read about the different ways this purple vegetable can improve your health.
https://medshadow.org/should-i-take-beetroot-supplements/

Visit us as AskaPatient.com for drug ratings and reviews provided by patients along with more news and health information.
If you like Drugs & Treatments News, please share it with a friend or click the “like” button so that more readers can find it! Thank you.