April 2, 2023: Drugs for rare skin cancer, eczema; label changes for Hyzaar, Fetzima, Vuity; ADHD stimulant use, overdiagnosis; the state of chronic pain treatment; cancer immunotherapy; allergy tips
FDA Drug Approvals
The U.S. Food & Drug Administration (FDA) approved Incyte's immunotherapy (monoclonal antibody) drug Zynyz (retifanlimab) to treat advanced cases of Merkel Cell carcinoma (MCC), a rare skin cancer. Zynyz is administered as an intravenous infusion for 30 minutes every 4 weeks for up to 24 months. Because the drug was granted accelerated approval, it requires follow-up studies to show clinical benefit. Serious adverse reactions occurred in 22% of trial patients given retifanlimab. Immunotherapy drugs Keytruda (pembrolizumab) (in 2019), and Bavencio (avulemab) (in 2017) have also been approved to treat MCC.
https://www.curetoday.com/view/fda-approves-zynyz-for-subset-of-patients-with-rare-skin-cancer
FDA Press Release
Pfizer's once-daily oral tablet for eczema, Cimbinqo (abrocitinib), may now be used to treat adolescents age 12 and up. The drug was originally approved for adults in January 2022.
https://www.msn.com/en-us/health/medical/pfizer-pfe-eczema-drug-cibinqo-gets-fda-nod-for-adolescents/ar-AA17qGbK
FDA label change
Allergan/AbbVie's Vuity eye drops prescription medicine for treating presbyopia, or age-related blurry near vision in adults, now has a twice-daily dosing option. The expanded approval allows for a second dose of the drop 3 to 6 hours after the first dose, which may extend the drop’s duration of effect for up to 9 hours. Vuity was originally approved in December 2021. It works by constricting the pupils.
https://www.healio.com/news/ophthalmology/20230330/fda-approves-twicedaily-dosing-option-of-vuity-for-presbyopia
A CBS Morning 4 minute video story from 2021 includes an interview with a patient who participated in the clinical trial of Vuity.
https://www.cbsnews.com/news/vuity-eye-drops-fda-approved-blurred-vision-presbyopia/
The FDA approved Emergent Biosolution's opioid overdose treatment Narcan nasal spray (naloxone hydrochloride) for sale over-the-counter. While it was already available without a prescription at some pharmacies, the Rx-to-OTC switch means now it can be sold at many kinds of stores, including retail locations like gas station convenience markets. Because of potentially high prices for the drug, however, it is not clear how many retail stores will want to offer it. For now, there is no generic alternative for the nasal spray, but there are other generic forms of injectable naloxone. Naloxone received FDA approval in 1971 for the treatment of overdoses. Narcan nasal spray was originally approved in 2015 as a prescription drug.
https://www.npr.org/2023/03/29/1166750095/narcan-fda-approval-naloxone-over-the-counter-ot

Drug Safety Updates
Hypertension drug Hyzaar (hydrochlorothiazide; losartan potassium) had a safety label update to warn that shortness of breath and difficulty breathing can signal an allergic reaction, and patients experiencing those symptoms should get emergency medical help right away and stop taking Hyzaar.
Hyzaar FDA safety labeling change
Hyzaar patient reviews from Ask a Patient
Antidepressant drug Fetzima (levomilnacipran hydrochloride), a serotonin and norepinephrine reuptake inhibitor (SNRI), had a safety label update to warn of elevated blood pressure adverse reactions. Blood pressure should be measured prior to initiating treatment and periodically throughout Fetzima treatment. The label was also updated with common adverse reactions and to state that it should not be used in pediatric populations.
Fetzima FDA safety labeling change
Fetzima patient reviews from Ask a Patient
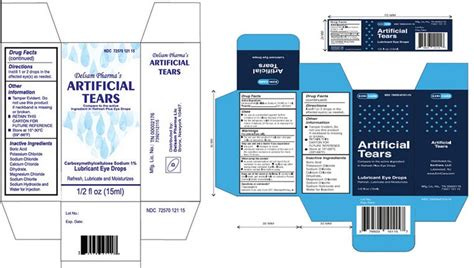
The Centers for Disease Control (CDC) reports that people continue to be sickened by the extremely drug-resistant bacteria called P. aeruginosa connected to some artificial tears products. As of March 14, 68 patients in 16 states (CA, CO, CT, FL, IL, NC, NJ, NM, NY, NV, PA, SD, TX, UT, WA, WI) have become ill. Thirty-seven of the patients were linked to four healthcare facility clusters. Three people have died, eight have had vision loss, and four cases required enucleation (surgical removal of eyeball). EzriCare Artificial Tears, a preservative-free, over-the-counter product is the brand most commonly reported being used by those who became sick. Patients and healthcare providers should immediately stop the use of EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears products.
https://www.cidrap.umn.edu/antimicrobial-stewardship/outbreak-highly-resistant-pseudomonas-linked-eye-drops-grows-68
CDC Press Release
ADHD Treatments
A CDC study found that the percentage of adolescent and adult females and adult males taking prescription stimulants for attention-deficit/hyperactivity disorder (ADHD) increased between 2016 and 2021. Notably, there was a sharp increase in patients taking stimulants between 2020 and 2021, which was attributed to expanded patient access to the drugs via telehealth during the pandemic. The percentage of females between ages 20 and 24 with at least one stimulant prescription grew from 5.2 to 6.2 percent between 2020 and 2021, the biggest change (a 19% annual percentage change) out of any age bracket in either sex group. Stimulant prescriptions were most common among boys, with 9.9 percent of boys ages 10 to 14 having at least one prescription in 2021.
The authors suggest that the uptick in prescription stimulants “raise questions about current adult ADHD care” and may merit more development on clinical recommendations for ADHD diagnoses and management in adults, as well as more evaluation of the benefits and harms of policies enacted during the pandemic. The study, "Trends in Stimulant Prescription Fills Among Commercially Insured Children and Adults — United States, 2016–2021" was published in Morbidity and Mortality Weekly Report (MMWR).
https://www.cdc.gov/mmwr/volumes/72/wr/mm7213a1.htm
The recent CDC research comes amid a continuing shortage of Adderall (amphetamine aspartate) a stimulant drug commonly prescribed for ADHD. Some generic manufacturers report backorders, while others cite shortage of active ingredients. In the FDA Drug Shortages Database, generic maker Teva notes that its Adderall tablets are available but that it continues to experience "unprecedented increase in demand."
https://www.marketwatch.com/story/amid-adderall-shortage-cdc-says-pandemic-may-have-led-to-or-exacerbated-adhd-symptoms-fcc25bf9
FDA Drug Shortages Database
American Society of Health-System Pharmacists Drug Shortages Database

Because stimulants like Adderall can be addictive or misused, some health plans, clinics, and state Medicaid programs require that patients be screened to check if they are safely taking their pills, and not selling them, taking too many, or using other drugs. Kaiser Health News investigated the disparities around testing requirements and found that some plans require urine tests several times a year, while others have no requirements.
Connecticut psychiatrist Dr. Margaret Chaplin noticed the lack of testing standards about eight years ago, when she and colleagues proposed ways to prevent stimulant misuse in adult ADHD patients. Her team recommended urine tests only if patients exhibit “red-flag behavior,” such as appearing intoxicated, repeatedly reporting lost prescriptions, or frequently switching doctors. A recent survey found that 42% of family physicians and 21% of college health professionals who treat adult ADHD require their patients to submit random urine drug screens.
https://khn.org/news/article/adhd-urine-testing-disparities-stigma-health-costs/
Join hosts Dr. Adriane Fugh-Berman and Caroline Renko for the latest "Pharmanipulation" podcast as they interview licensed pediatric psychologist Dr. Gretchen LeFever Watson and journalist Robert Whitaker (from Mad in America) about the overdiagnosis of ADHD. The conversation covers such topics as:
The history of ADHD diagnosis, which began in the 1980s with the third edition of Diagnostic and Statistical Manual of Mental Disorders (DSM-3).
Problems with subjective "evaluation scorecards:" can the condition be reliably diagnosed with a scorecard? To this day, there is no "test" for ADHD.
Can a preschooler really have ADHD?
What are the outcomes for the kids that "enter the system of treatment" vs. those that don't?
Why some teachers have been trained on how to identify those kids that might need drug stimulant treatment rather than how to change the classroom learning environment itself.
The program page includes links to resources, research studies and papers that are discussed in the interview. The podcast is produced by PharmedOut, a Georgetown University Medical Center "rational prescribing project."
Podcast Episode 3

Pain Treatments
In "Chronic Pain: the Long Road to Discovery" Nature's Lucy Odling-Smee, herself a sufferer of chronic back pain, interviews and photographs two people who live their lives in pain and tells the stories of how they cope. Although new treatments are being explored and are viable, millions of people with chronic pain are still without help.
https://www.nature.com/immersive/d41586-023-00869-6/index.html
This PDF version of the article provides a read with less scrolling: https://media.nature.com/original/magazine-assets/d41586-023-00869-6/d41586-023-00869-6.pdf
Potential New Paradigm for Cancer Treatment
Could a new paradigm for cancer treatment be emerging? A preliminary study by MD Anderson suggests that for some people with early-stage cancer, 6 months of immunotherapy or just 4 weeks of immunotherapy followed by minor surgery could be the only treatment they might ever need. The study was published in Journal of Clinical Oncology.
https://www.cancer.gov/news-events/cancer-currents-blog/2023/neoadjuvant-immunotherapy-only-treatment
Allergy Medication Tips
Can you safely take allergy drugs if you have high blood pressure?
According to this pharmacist, it's ok to take Benadryl or loratadine or cetirizine (brands Claritin or Zyrtec) but not Claritin-D or Zyrtec-D because those contain the ingredient pseudoephedrine, which might raise blood pressure.
https://www.singlecare.com/blog/does-benadryl-raise-blood-pressure/
If you don't get relief from your allergy medication, can you take two at the same time, such as cetirizine and Benadryl or some other combination? A medical doctor and pharmacist explain the consequences of mixing allergy medications and provide guidance for situations when it may be ok to do so.
https://www.singlecare.com/blog/mixing-allergy-medicine/
Read and add patient drug ratings and reviews at www.askapatient.com.
